Highlight
• Increasing daily sit-to-stand transitions by approximately 25 times per day significantly decreases diastolic blood pressure in overweight and obese postmenopausal women.
• Reducing total sitting time by nearly one hour daily did not elicit significant improvements in blood pressure or glucose regulation.
• Sitting time and sit-to-stand transitions represent distinct, non-interchangeable behavioral targets.
Background
Postmenopausal women are a population at elevated risk for cardiovascular diseases, diabetes, and related morbidities due largely to high sedentary behavior characterized by prolonged sitting. Despite traditional emphasis on increasing moderate to vigorous physical activity (MVPA), only 3–6% of adults over 60 achieve recommended levels of MVPA, indicating a need for alternative behavioral interventions that are feasible and acceptable. Sedentary behavior modifications, specifically reducing total sitting time or increasing the frequency of sit-to-stand transitions (STST), have emerged as promising strategies for improving cardiometabolic health. Prior studies supporting these interventions have often been limited by small sample sizes, short intervention durations, and non-targeted populations. The Rise for Health trial uniquely addresses these gaps by evaluating two distinct interventions—reducing overall sitting versus increasing STST—and their differential impacts on blood pressure and glucose regulatory biomarkers in a large cohort of overweight/obese postmenopausal women.
Study Design
This randomized controlled trial enrolled 407 sedentary, overweight or obese postmenopausal women aged 55 years or older from the University of California San Diego between 2018 and 2022. Participants met criteria of ≥7 hours daily sitting and ≤70 STSTs per day at baseline, among other health criteria ensuring safety. They were randomized 1:1:1 to one of three 3-month intervention arms: (1) Healthy Living control; (2) Sit Less—targeting reduction in total sitting time; or (3) Sit-to-Stand—increasing the number of daily sit-to-stand transitions. Both intervention arms received seven individual behavioral coaching sessions utilizing habit formation, social cognitive theory, and motivational interviewing. Objective measures included accelerometer-based assessments (activPAL and ActiGraph) of sitting behavior and MVPA, and physiological assessments of fasting blood insulin, glucose, HbA1c, HOMA2-IR, and resting blood pressure at baseline and 3 months. The primary outcomes were composite z-scores for blood pressure and glucose regulatory biomarkers.
Key Findings
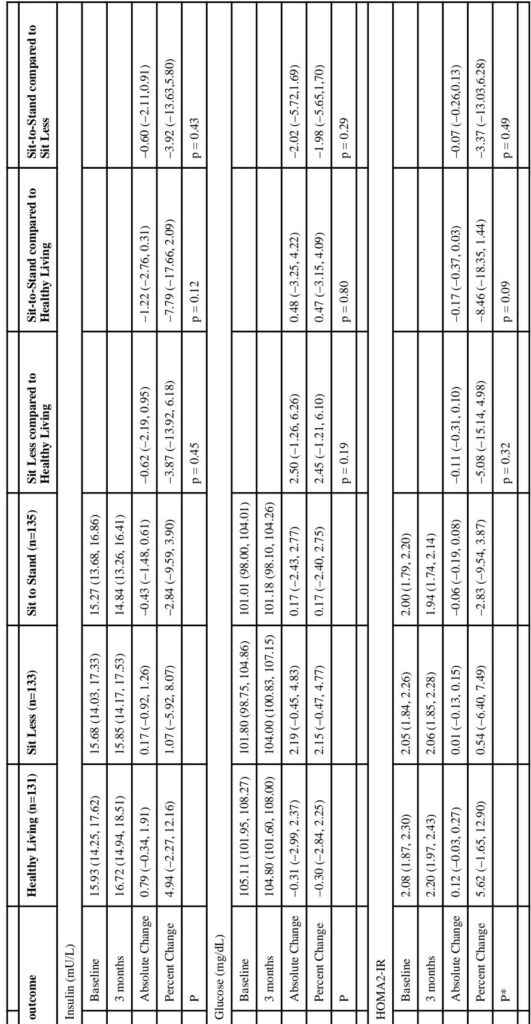
Retention was high (95%), with 388 women completing the trial. The Sit Less arm achieved a significant reduction in total sitting time of 58 minutes per day relative to the control group (95% CI -83 to -34 minutes, p < 0.001) but showed no significant change in STST. Conversely, the Sit-to-Stand arm increased STST by approximately 26 transitions per day relative to controls (95% CI 18 to 34 transitions, p < 0.001) without significantly reducing sitting time.
Regarding physiological outcomes, the Sit-to-Stand arm demonstrated significant reductions in diastolic blood pressure (-2.24 mmHg; 95% CI -4.08 to -0.40; p = 0.02) compared to controls, and a non-significant trend toward reduced systolic blood pressure (-3.33 mmHg; p = 0.03) though not meeting the stricter alpha threshold of 0.025. These findings persisted after adjustment for MVPA changes. The Sit Less arm showed non-significant decreases in both systolic and diastolic blood pressures. No significant changes were observed in fasting glucose, insulin, HbA1c, or insulin resistance indices in either intervention arm compared to controls.
Adverse events were minor, predominantly mild skin irritation from monitoring devices, and musculoskeletal complaints related to the interventions.
Expert Commentary
This trial reinforces the conceptual distinction between total sitting duration and sit-to-stand transitions as separate behavioral constructs with distinct physiological impacts. The observed blood pressure improvement in response to increased STST aligns with mechanistic hypotheses that frequent postural shifts enhance vascular shear stress, endothelial function, and muscle activation, potentially attenuating hypertension. The absence of glucose regulatory improvements may reflect the relatively short intervention duration or inclusion of participants without overt glucose dysregulation; longer trials or more targeted populations may be necessary to capture glycemic benefits. Additionally, the improvement magnitude, though modest, could have meaningful public health implications when scaled population-wide.
Limitations include the predominantly white, educated sample limiting generalizability, absence of dietary and sleep variables, and the reliance on fasting rather than continuous glucose measurements for metabolic assessment. Future investigations should test extended intervention durations, diverse populations including men, and explore combined sitting reduction and STST increases.
Conclusion
The Rise for Health trial demonstrates that among overweight and obese postmenopausal women, behavioral interventions focused on increasing sit-to-stand transitions can effectively reduce diastolic blood pressure within three months, whereas reducing total sitting time alone does not. These findings suggest that targeting sit-to-stand transitions may be a practical and physiologically impactful strategy to mitigate cardiovascular risk in this vulnerable group. Clinicians should consider counseling patients to increase the frequency of positional changes from sitting to standing as part of cardiovascular risk reduction strategies. Further research is warranted to elucidate long-term effects and metabolic outcomes.
References
Hartman SJ, LaCroix AZ, Sears DD, Natarajan L, Zablocki RW, Chen R, Patterson JS, Dillon L, Sallis JF, Schenk S, Dunstan DW, Owen N, Rosenberg DE. Impacts of Reducing Sitting Time or Increasing Sit-to-Stand Transitions on Blood Pressure and Glucose Regulation in Postmenopausal Women: Three-Arm Randomized Controlled Trial. Circulation. 2025 Aug 26;152(8):492-504. doi: 10.1161/CIRCULATIONAHA.124.073385. Epub 2025 Jul 25. PMID: 40709462; PMCID: PMC12342652.