Highlights
- Routine Helicobacter pylori (H. pylori) screening during hospitalization for myocardial infarction (MI) did not significantly decrease the incidence of upper gastrointestinal bleeding (UGIB).
- Approximately 70% of patients were screened, with a 23.6% positivity rate for H. pylori infection.
- Subgroup analyses indicated potential benefits of screening in patients with anemia and those with impaired kidney function.
- Current evidence advises against universal H. pylori screening post-MI, but targeted risk-stratified screening strategies may be warranted.
Background
Despite significant advancements in acute myocardial infarction care, bleeding complications remain a notable concern, particularly upper gastrointestinal bleeding (UGIB), which is the most common bleeding site post-MI and is associated with increased mortality and cardiovascular adverse outcomes. Standard antithrombotic regimens employed for secondary prevention in MI patients include potent antiplatelet agents and anticoagulants, which elevate UGIB risk.
Proton pump inhibitors (PPIs) have been shown to reduce UGIB risk and are sometimes prescribed prophylactically, though their long-term routine use remains controversial due to safety concerns and cost-effectiveness considerations.
Helicobacter pylori, a common cause of chronic active gastritis, is prevalent among cardiovascular patients and can exacerbate UGIB risk, especially when combined with antithrombotic therapy. While gastroenterological guidelines recommend screening and eradication of H. pylori in long-term aspirin users to mitigate bleeding risk, cardiology guidelines have not widely adopted this recommendation, leaving a clinical management gap.
The HELP-MI SWEDEHEART trial was designed to evaluate whether routine H. pylori screening during hospitalization for MI could reduce the incidence of UGIB events, thereby informing clinical practice and interdisciplinary guidelines.
Study Design
The HELP-MI SWEDEHEART trial was a nationwide, open-label, two-period, two-sequence cluster randomized crossover trial conducted in Sweden involving 35 hospitals grouped into 18 clusters. The intervention entailed alternating between 1 year of routine H. pylori screening using the 13C-urea breath test during MI hospitalization and 1 year of usual care without screening, separated by a 2-month washout interval. The trial enrolled adult patients discharged alive after type 1 MI registered in the SWEDEHEART registry from November 17, 2021, to January 17, 2024, with follow-up until January 17, 2025.
The primary endpoint was the incidence of upper gastrointestinal bleeding (UGIB), analyzed using an intention-to-treat framework with a negative binomial regression model to estimate rate ratios. Secondary endpoints included net adverse clinical events, cardiovascular events, and all-cause and cardiovascular mortality.
Key Findings
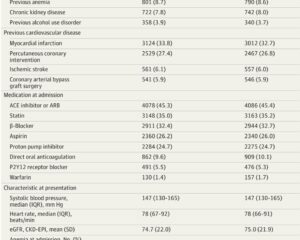
A total of 18,466 patients participated, with a median age of 71 years and 71% male representation. In the screening arm, 9245 patients were eligible for H. pylori testing; compliance was about 70%, and among those tested, 23.6% were positive for H. pylori infection. Almost all H. pylori-positive patients (96.6%) received eradication therapy based on physician discretion.
Over a median follow-up of 1.9 years, 299 UGIB events occurred in the screening group, yielding an incidence rate of 16.8 per 1000 person-years versus 336 events (19.2 per 1000 person-years) in the usual care group. The difference was not statistically significant (rate ratio 0.90; 95% CI, 0.77–1.05; P = .18).
Secondary outcomes, including composite clinical events and mortality (all-cause and cardiovascular), showed no significant differences between groups.
Subgroup Analyses
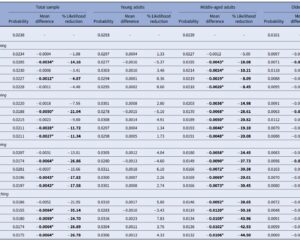
- Anemia Status: Patients with mild anemia (hemoglobin 100–120 g/L) exhibited a significant reduction in UGIB risk with screening (RR 0.64), and a more pronounced reduction was observed in those with moderate to severe anemia (hemoglobin <100 g/L; RR 0.44), with statistically significant interaction (P for interaction = .03).
- Kidney Function: Patients with impaired renal function (eGFR <60 mL/min/1.73 m2) benefited more from screening (RR 0.75) compared to those with preserved renal function.
- PPI Use: Among patients discharged without PPI therapy, screening correlated with a significant UGIB risk reduction, highlighting potential interaction effects between H. pylori eradication and PPI prophylaxis.
Per-protocol analyses suggested a trend towards greater UGIB risk reduction among patients who tested positive and completed eradication therapy; however, these did not reach statistical significance, potentially owing to limited power.
Expert Commentary
The HELP-MI SWEDEHEART trial provides a methodologically robust, pragmatic appraisal of routine H. pylori screening following acute MI in a large unselected population with real-world generalizability, utilizing high-quality national registries (SWEDEHEART) and a cluster randomized crossover design to mitigate confounding.
Several factors may contribute to the overall neutral primary outcome:
- Lower-than-expected H. pylori prevalence (23.6% versus the anticipated ~30%) may reduce potential population-level impact.
- Incomplete screening adherence (~70%) introduces dilution bias.
- Widespread PPI use (~25% on admission, ~50% at discharge) could reduce UGIB risk generally and impair urea breath test sensitivity, potentially causing false-negative results.
The subgroup benefits in anemic and renally impaired patients suggest that risk stratification may identify populations most likely to benefit from targeted screening and eradication. This aligns with pathophysiological rationales wherein anemia denotes higher bleeding vulnerability and kidney disease impairs mucosal defense, increasing bleeding risk.
Limitations include reliance on administrative ICD coding for UGIB events without centralized adjudication, potential underestimation of effects due to incomplete screening and adherence, and lack of systematic follow-up to confirm eradication success post-discharge. The crossover design risk of period effects was mitigated by a washout period but cannot be fully excluded.
Comparison with prior evidence, including the HEAT trial which showed transient UGIB reduction after H. pylori eradication in aspirin users, extends understanding to the broader post-MI population receiving multi-agent antithrombotic therapy.
Conclusion
Routine universal H. pylori screening during MI hospitalization does not significantly reduce subsequent upper gastrointestinal bleeding events. However, selective screening targeting high-risk subgroups such as patients with anemia or kidney failure may improve clinical outcomes. Integrating risk stratification tools into post-MI management protocols could optimize bleeding prevention strategies and tailor H. pylori eradication therapy accordingly.
These findings recommend against universal screening in current practice but encourage personalized approaches, further research into optimizing screening modalities under PPI therapy, and guideline updates to reflect nuanced patient risk profiles.
References
- Hofmann R, James S, Sundqvist MO, Wärme J, Angerås O, Alfredsson J, Erlinge D, Arefalk G, Arstad G, Blomberg S, Fröbert O, Hambraeus K, Hellström PM, Lauermann J, Lidin M, Lindhagen L, Mourtzinis G, Schoede C, Thunström E, Voldberg B, Wagner H, Östlund O, Jernberg T, Bäck M. Helicobacter pylori Screening After Acute Myocardial Infarction: The Cluster Randomized Crossover HELP-MI SWEDEHEART Trial. JAMA. 2025 Sep 1. doi:10.1001/jama.2025.15047. PMID: 40887995.