Highlight
- The Score of Integrated Balance in Ataxia (SIBA) is a novel composite digital measure of static and dynamic balance derived from wearable inertial sensors in patients with spinocerebellar ataxia (SCA).
- SIBA demonstrates excellent test-retest reliability, high validity against the established Scale for the Assessment and Rating of Ataxia (SARA), and strong discriminative power between ataxic patients and healthy controls.
- Compared to SARA, SIBA detects disease progression with a larger effect size and requires substantially fewer participants in clinical trials to demonstrate treatment effects.
- These findings suggest that SIBA could facilitate faster, smaller, and more efficient clinical trials targeting balance impairment in SCA.
Study Background
Spinocerebellar ataxias (SCAs) are a group of genetically heterogeneous, progressive neurodegenerative disorders primarily affecting cerebellar function. Characterized by gait and balance impairment, they lead to increasing disability and fall risk. Despite growing therapeutic interest, clinical trials for SCAs remain challenging due to the slow progression and variability of symptoms, requiring large sample sizes to detect meaningful treatment effects using existing clinical scales such as the Scale for the Assessment and Rating of Ataxia (SARA).
The unmet need for more sensitive, objective, and quantitative endpoints has driven research toward digital biomarkers. Wearable inertial sensors provide rich kinematic data on gait and balance, offering a potential avenue to develop composite metrics with improved sensitivity and reliability. The Score of Integrated Balance in Ataxia (SIBA) was developed to address this need by integrating multiple facets of static and dynamic balance into a single objective score.
Study Design
This prospective study involved two phases: a development phase using a retrospective cohort, and a validation phase with an independent prospective sample. Participants were genetically confirmed adults aged 18 to 75 years with SCA types 1, 2, 3, or 6, recruited from clinical sites in the United States and Cuba.
Eligibility criteria included the ability to walk 10 meters independently without assistive devices and to stand unassisted for 30 seconds. Age- and sex-matched healthy controls, mostly family members, were recruited for comparison.
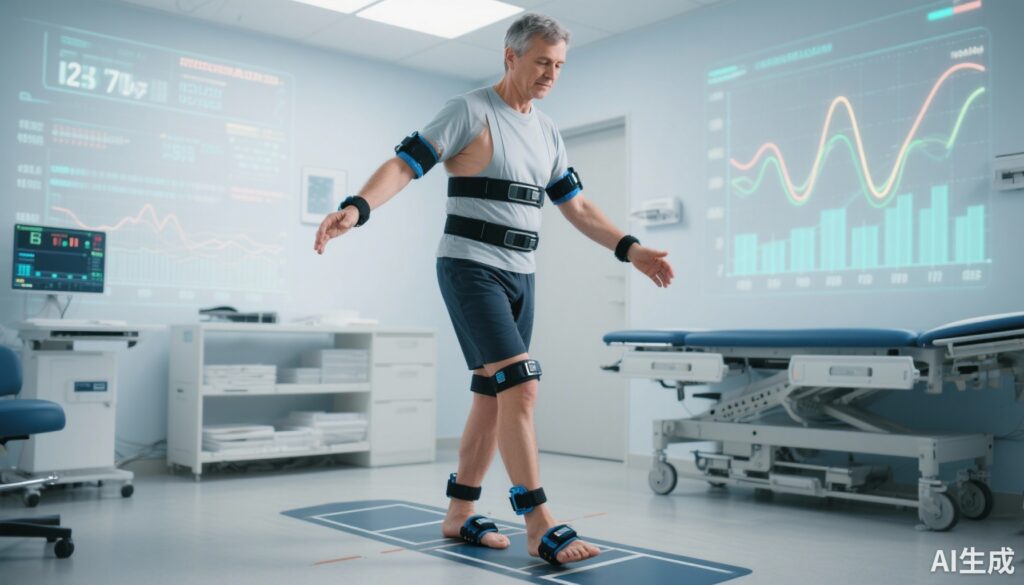
Balance and gait assessments were conducted using six wearable Opal inertial measurement units placed bilaterally on the dorsum of each foot and hand, sternum, and lower lumbar vertebral segment. Participants performed a 2-minute natural pace walk and static stance tests (feet together and apart for 30 seconds each).
The SIBA was constructed through a multiple criteria decision analysis, weighting variables based on discriminatory power, reliability, validity, progression sensitivity, and clinical meaningfulness. The final composite combined four parameters: variability of toe-out angle and double-support time proportion (dynamic balance), plus sway angle root mean square (RMS) and sway acceleration RMS (static balance).
Key Findings
The study included 258 individuals (131 females, 127 males; 40 premanifest, 218 ataxic) and 100 healthy controls in the development phase, and 53 individuals with SCA plus 24 controls in the validation cohort.
SIBA showed strong concurrent validity with SARA (Pearson’s r=0.736), indicating close alignment with the established clinical scale. Test-retest reliability was excellent (intraclass correlation coefficient=0.970), supporting its reproducibility.
Crucially, SIBA distinguished SCA patients from controls with high accuracy (AUROC=0.956) and predicted fall risk with fair discrimination (AUROC=0.760). Over one year, SIBA detected disease progression with an effect size of 0.59, substantially larger than SARA’s 0.11, underscoring superior sensitivity.
Sample size projections indicated that clinical trials using SIBA could require 88% fewer participants than those relying on SARA to detect a 50% reduction in disease progression rate over 12 months (171 vs 1491 participants), highlighting great efficiency gains.
Expert Commentary
SIBA represents a timely advancement in quantitative ataxia assessment. By leveraging wearable sensor technology and composite analytics, it provides a reliable, valid, and sensitive measure of balance deficits that reflect core cerebellar dysfunction in SCA.
The incorporation of both static and dynamic balance elements captures multiple dimensions of motor control, which are essential for comprehensive ataxia evaluation. Its ability to predict fall risk adds clinical relevance beyond traditional rating scales.
Limitations include the current focus on ambulatory individuals who can walk independently and stand unassisted, potentially limiting generalizability to more severely affected patients. Further studies are needed to assess responsiveness to interventions and applicability in diverse clinical settings.
Additionally, integration into clinical trials will require validation across larger international cohorts and exploration of digital biomarker standardization.
Conclusion
The Score of Integrated Balance in Ataxia (SIBA) offers a robust, sensitive, and efficient digital biomarker for assessing balance in spinocerebellar ataxias. Its superior sensitivity to progression and reduced sample size needs could accelerate clinical trial development and enhance therapeutic evaluation in these devastating disorders.
Future research should focus on confirming responsiveness to therapeutic interventions, expanding validation to broader patient populations, and integrating SIBA into multi-modal clinical trial endpoints.
Funding and Clinical Trial Registration
This study was supported by Biogen, Clario, Pfizer, and the Alexander von Humboldt Foundation. The validation cohort was registered under ClinicalTrials.gov identifier NCT04268147.
Reference
McNames J, Shah VV, Casey HL, et al. Development and validation of a composite digital balance score for spinocerebellar ataxia: a prospective study. Lancet Digit Health. 2025 Oct 16;100905. doi: 10.1016/j.landig.2025.100905. Epub ahead of print. PMID: 41107202.