Highlights
- Retifanlimab, when added to carboplatin and paclitaxel, significantly improved progression-free survival (PFS) in patients with advanced squamous cell carcinoma of the anal canal (SCAC).
- The combination therapy demonstrated a manageable safety profile, with adverse event rates comparable to standard chemotherapy.
- This phase 3 trial supports retifanlimab plus carboplatin-paclitaxel as a new standard of care for first-line treatment in advanced SCAC.
Study Background and Disease Burden
Squamous cell carcinoma of the anal canal (SCAC) is a rare but increasingly prevalent malignancy, often associated with human papillomavirus (HPV) infection. Advanced or metastatic cases have poor prognosis, with limited therapeutic options after progression on platinum-based chemotherapy. Historically, standard first-line treatment for inoperable locally recurrent or metastatic SCAC consisted of carboplatin and paclitaxel, as established by the InterAACT trial. However, outcomes remain unsatisfactory, with a clear unmet need for more effective therapies. Immunotherapy targeting the PD-1/PD-L1 pathway has shown promise in several epithelial cancers, including SCAC, particularly in the setting of disease progression. Retifanlimab, a PD-1 inhibitor, has demonstrated clinical activity in PD-L1-positive advanced SCAC previously treated with platinum regimens. The POD1UM-303/InterAACT-2 trial was designed to assess whether integrating retifanlimab with standard carboplatin-paclitaxel as initial therapy could improve clinical outcomes in this high-risk population.
Study Design
POD1UM-303/InterAACT-2 is a global, multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Conducted across 70 centres in 12 countries, including the EU, Australia, Japan, the UK, and the USA, the trial enrolled adults (≥18 years) with inoperable locally recurrent or metastatic SCAC, ECOG performance status 0 or 1, no previous systemic therapy, and (if HIV-positive) well-controlled infection (CD4+ >200/μL, undetectable viral load). Eligible patients (n=308) were randomised 1:1 to receive either retifanlimab (500 mg intravenous every 4 weeks) or placebo, each in combination with standard carboplatin-paclitaxel, for up to 1 year. Upon confirmed disease progression, placebo group patients could cross over to retifanlimab monotherapy. The primary endpoint was independently assessed progression-free survival (PFS) by RECIST v1.1. Secondary endpoints included overall survival, objective response rate, and safety. Efficacy analyses were conducted by intention to treat.
Key Findings
Between November 2020 and July 2023, 308 patients were randomised equally to the two arms. The population was predominantly female (72%) and included patients from diverse geographic regions. The addition of retifanlimab yielded a clinically and statistically significant improvement in PFS:
– Median PFS: 9.3 months (95% CI 7.5-11.3) in the retifanlimab arm vs 7.4 months (7.1-7.7) in the placebo arm.
– Hazard ratio (HR) for progression or death: 0.63 (95% CI 0.47-0.84), with a one-sided p=0.0006, indicating a 37% reduction in the risk of progression or death.
Serious and grade 3 or higher adverse events were more frequent in the retifanlimab group (47.4% and 83.1%) than in the placebo group (38.8% and 75.0%). The most common grade ≥3 events were neutropenia (35.1% vs 29.6%) and anaemia (19.5% vs 20.4%). Four fatal adverse events occurred in the retifanlimab arm (only one considered treatment-related: pancytopenia), and one in the placebo arm (not treatment-related).
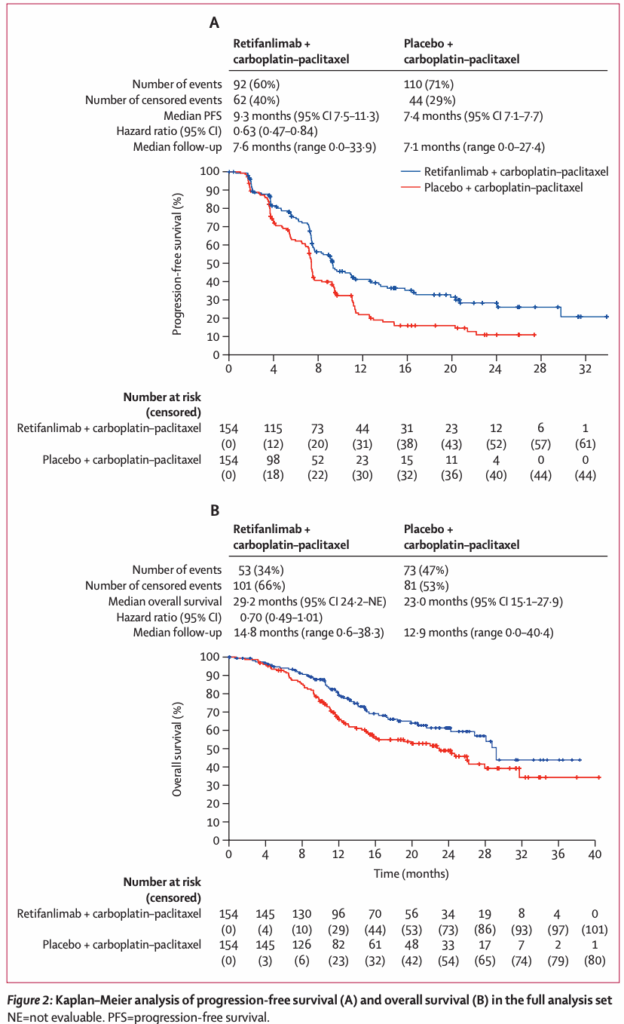
Although data on overall survival (OS) were not mature at the time of reporting, the observed PFS benefit and manageable toxicity profile mark a clinically meaningful advance. The trial’s crossover design may attenuate OS differences, a common challenge in immunotherapy trials.
Expert Commentary
The addition of retifanlimab to carboplatin-paclitaxel represents the first major advancement in first-line therapy for advanced SCAC in over a decade. The magnitude of PFS improvement (nearly two months) is notable in a disease with historically limited options. The safety profile, while showing increased rates of high-grade adverse events, remains consistent with expectations for immune checkpoint inhibitors and cytotoxic chemotherapy.
Expert opinion, as reflected in recent editorials and guideline commentaries, emphasizes the importance of this trial in setting a new standard of care. The trial’s inclusion of patients with well-controlled HIV infection enhances generalizability to a key at-risk population. Limitations include the absence of mature OS data and the higher toxicity burden, which necessitates vigilant patient monitoring. Additionally, the crossover design, while ethically appropriate, may obscure long-term survival benefits attributable to initial combination therapy.
Mechanistically, retifanlimab’s activity is biologically plausible given the immunogenic nature of HPV-related SCAC and the established efficacy of PD-1 blockade in similar tumor types. The integration of immunotherapy early in the treatment course, rather than as salvage, aligns with evolving paradigms in solid tumor oncology.
Conclusion
The POD1UM-303/InterAACT-2 trial establishes retifanlimab plus carboplatin-paclitaxel as a new, evidence-based first-line regimen for inoperable locally recurrent or metastatic SCAC. The improvement in progression-free survival, coupled with a manageable safety profile, supports immediate integration into clinical practice. Ongoing follow-up for overall survival and further real-world studies will inform the long-term impact and optimal patient selection for this regimen. Clinicians should consider immune-related toxicity monitoring and multidisciplinary management to maximize patient benefit.
References
1. Rao S, Samalin-Scalzi E, Evesque L, Ben Abdelghani M, Morano F, Roy A, Dahan L, Tamberi S, Dhadda AS, Saunders MP, Casanova N, Guimbaud R, Lievre A, Maurel J, Fakih M, Tian C, Harrison J, Jones MM, Cornfeld M, Spano JP, Rochefort P; POD1UM-303/InterAACT-2 study investigators. Retifanlimab with carboplatin and paclitaxel for locally recurrent or metastatic squamous cell carcinoma of the anal canal (POD1UM-303/InterAACT-2): a global, phase 3 randomised controlled trial. Lancet. 2025 Jun 14;405(10495):2144-2152. doi: 10.1016/S0140-6736(25)00631-2 IF: 88.5 Q1 . PMID: 40517007 IF: 88.5 Q1 .
2. Kim S, et al. Immunotherapy in the management of anal cancer: From current evidence to future directions. J Immunother Cancer. 2023;11(7):e006745.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Anal Carcinoma. Version 2.2024. Accessed June 2024.