Study Background and Disease Burden
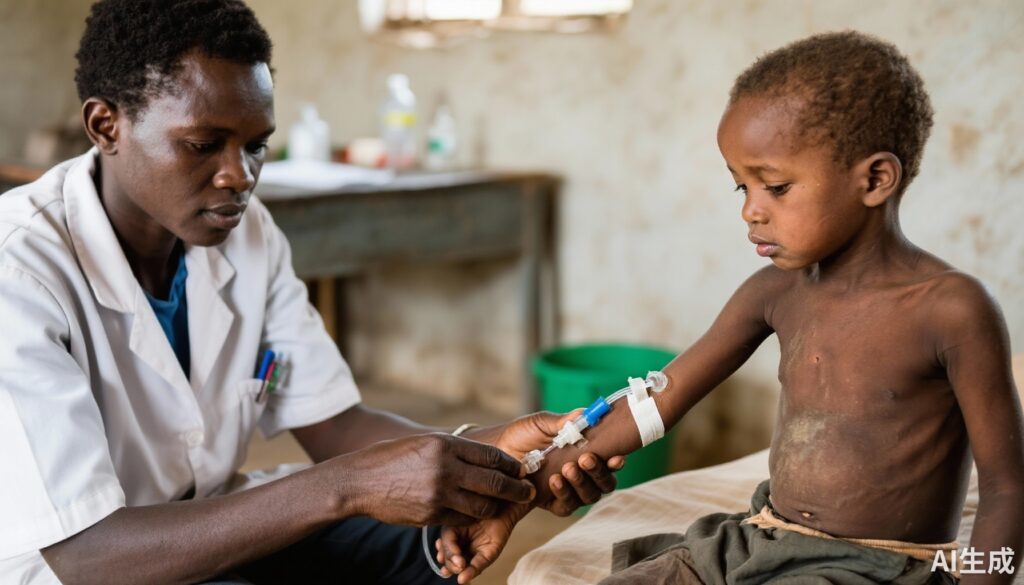
Severe acute malnutrition (SAM) remains a major contributor to childhood morbidity and mortality in low-resource settings, particularly sub-Saharan Africa. Gastroenteritis often complicates SAM by causing significant dehydration, which necessitates prompt rehydration to reduce fatal outcomes. Current international guidelines caution against intravenous (IV) fluid administration in children with SAM due to concerns of fluid overload, heart failure, and pulmonary edema, despite the fact that supportive evidence for these concerns is limited. Consequently, oral rehydration therapy (ORT) is favored as the primary modality, even though its efficacy may be compromised by gastrointestinal dysfunction or shock. The persistent high mortality rates in this population call for re-evaluation of optimal rehydration strategies, including the potential role of intravenous fluids. This context motivated the GASTROSAM trial to assess safety and efficacy of intravenous rehydration compared with oral strategies in children with SAM and gastroenteritis-induced dehydration.
Study Design
This multicenter, open-label, factorial, superiority randomized controlled trial was conducted across four African countries involving children aged 6 months to 12 years diagnosed with severe acute malnutrition complicated by gastroenteritis and dehydration. Participants were randomized in a 2:1:1 ratio into three groups:
1. Oral rehydration strategy (oral rehydration plus intravenous boluses for shock as needed).
2. Rapid intravenous rehydration strategy using lactated Ringer’s solution at 100 ml/kg over 3 to 6 hours, with boluses for shock.
3. Slow intravenous rehydration strategy using lactated Ringer’s solution at 100 ml/kg over 8 hours, without boluses.
The primary endpoint was mortality at 96 hours post-intervention. Secondary outcomes included mortality at 28 days, serious adverse events, and evaluation of fluid overload complications such as pulmonary edema or heart failure. Nasogastric tube use was permitted for administration when required to facilitate oral rehydration.
Key Findings
A total of 272 children were randomized: 138 to the oral strategy, 67 to the rapid intravenous group, and 67 to the slow intravenous group. Follow-up extended to 28 days. Nasogastric tube administration was employed in 93% of oral group participants and 65% in intravenous groups, reflecting pragmatic administration challenges.
At 96 hours, mortality rates were statistically indistinguishable across groups: 8% (11/138) for oral rehydration and 7% (9/134) in intravenous strategies combined (5 in rapid IV and 4 in slow IV). The risk ratio for death at 96 hours between oral and intravenous approaches was 1.02 (95% CI, 0.41 to 2.52; P = 0.69), indicating no significant mortality benefit or harm from intravenous fluid administration.
The 28-day mortality was also comparable: 12% (17/138) in the oral group and 10% (14/134) in intravenous groups, with a hazard ratio of 0.85 (95% CI, 0.41 to 1.78). Secondary serious adverse events occurred more frequently in the oral rehydration group (23%) than in rapid (21%) or slow intravenous groups (15%), although this difference was not statistically emphasized.
Crucially, no clinical or radiological evidence of pulmonary edema, congestive heart failure, or fluid overload was observed across any groups, challenging the prevailing assumption that intravenous rehydration risks precipitating harmful volume-related complications in children with SAM.
Intravenous boluses for shock were judiciously used, occurring in a minority: 9% of oral group participants and 10% in the rapid intravenous group; no boluses were administered in the slow intravenous arm. This practice aligned with the trial’s safety-focused protocol and reflects real-world clinical decision-making.
These findings collectively provide robust evidence that intravenous rehydration, administered either rapidly or slowly, does not confer increased mortality nor marked safety risks compared with the standard oral rehydration strategies in children with SAM complicated by dehydration and gastroenteritis.
Expert Commentary
The GASTROSAM trial addresses a critical and previously underexplored dilemma in the management of dehydration in children with severe acute malnutrition. The long-standing caution against intravenous fluids stems mainly from clinical dogma rather than high-quality trial evidence. These new data suggest that with careful monitoring and appropriate fluid administration protocols, intravenous rehydration can be as safe as oral methods.
Nevertheless, the study’s open-label design and the heterogeneous clinical contexts across four countries limit some generalizability. Additionally, the trial excluded children with cardiogenic or other complex pathologies potentially predisposing to fluid overload, and thus caution remains warranted in such subpopulations.
The trial importantly emphasizes the role of nasogastric tubes for oral rehydration, underlining practical hurdles in SAM with gastroenteritis due to vomiting or gut dysfunction. The balance of risks and benefits may shift according to clinical acuity, concurrent shock, and staff expertise.
Current WHO guidelines may require re-examination in light of this evidence. Clinicians should weigh intravenous rehydration as a viable option when oral rehydration is unsafe or ineffective, especially in resource-limited settings with high mortality burden. Further mechanistic research into fluid distribution and cardiac function in malnourished children could elucidate underlying factors driving safety.
The trial serves to advance evidence-based protocols and encourage prospective refinements in global pediatric malnutrition management guidelines.
Conclusion
The GASTROSAM trial provides compelling evidence that intravenous rehydration strategies—both rapid and slow infusion of lactated Ringer’s solution—do not increase short-term mortality or fluid overload risks compared with oral rehydration approaches in children with severe acute malnutrition complicated by gastroenteritis-induced dehydration. These findings challenge entrenched clinical dogma, suggesting intravenous fluids are a safe therapeutic option when carefully administered and monitored.
This offers a potential paradigm shift in rehydration management for this vulnerable population, potentially improving outcomes where oral rehydration is insufficient or contraindicated. Further guideline updates and additional research on individualized fluid management are warranted to optimize care. Clinicians working in pediatric infectious diseases and malnutrition in resource-limited settings should consider these findings in context of their comprehensive clinical assessment.
Overall, the study’s evidence supports expanding the toolkit for lifesaving fluid therapy in children with SAM to include cautious use of intravenous rehydration without undue fear of fluid-related complications.
References
1. Maitland K, Ouattara SM, Sainna H, et al; GASTROSAM Trial Group. Intravenous Rehydration for Severe Acute Malnutrition with Gastroenteritis. N Engl J Med. 2025;393(13):1257-1268. doi:10.1056/NEJMoa2505752
2. World Health Organization. Guidelines for the inpatient treatment of severely malnourished children. Geneva: WHO; 2013.
3. Ahmed T, Mahfuz M, Islam MM, et al. Severe acute malnutrition among under-five children: Current status and future directions. J Paediatr Child Health. 2018;54(12):1255-1260. doi:10.1111/jpc.14169
4. Newton KP, Mucunguzi A, Tumwine JK, et al. Clinical determinants of mortality among hospitalized children with severe acute malnutrition: A systematic review and meta-analysis. Lancet Glob Health. 2023;11(3):e313-e323. doi:10.1016/S2214-109X(22)00408-0