Highlight
- Iodine-impregnated adhesive drapes halve the rate of pocket site bacterial contamination during repeat cardiac implantable electronic device (CIED) procedures.
- Use of iodine drapes eliminated adjudicated CIED infections in the treated group over one year of follow-up, compared with 1.9% infection rate in controls.
- Positive swab cultures correlate with a higher, though not statistically significant, risk for subsequent CIED infections, emphasizing early contamination’s role in pathogenesis.
- This low-cost, easy-to-implement intraoperative step could substantially decrease infection-related morbidity and healthcare costs in CIED replacements.
Study Background and Disease Burden
Cardiac implantable electronic devices (CIEDs), including pacemakers and defibrillators, are critical in managing arrhythmias and preventing sudden cardiac death. As device longevity extends and patient survival improves, repeat procedures—such as generator replacements, upgrades, or revisions—are increasingly common. Unfortunately, CIED infections remain a serious complication with substantial morbidity, mortality, and healthcare cost implications. Infection risk in repeat surgeries is notably higher than in first implants due to factors such as disrupted tissue planes and previous pocket colonization. One major pathophysiological mechanism driving these infections is contamination of the device pocket during surgery. Contaminated surgical fields facilitate bacterial biofilm formation on the device, which resists eradication and necessitates device removal in many cases. Therefore, interventions focused on minimizing pocket contamination during implantation are crucial in optimizing patient outcomes and resource utilization.
Study Design
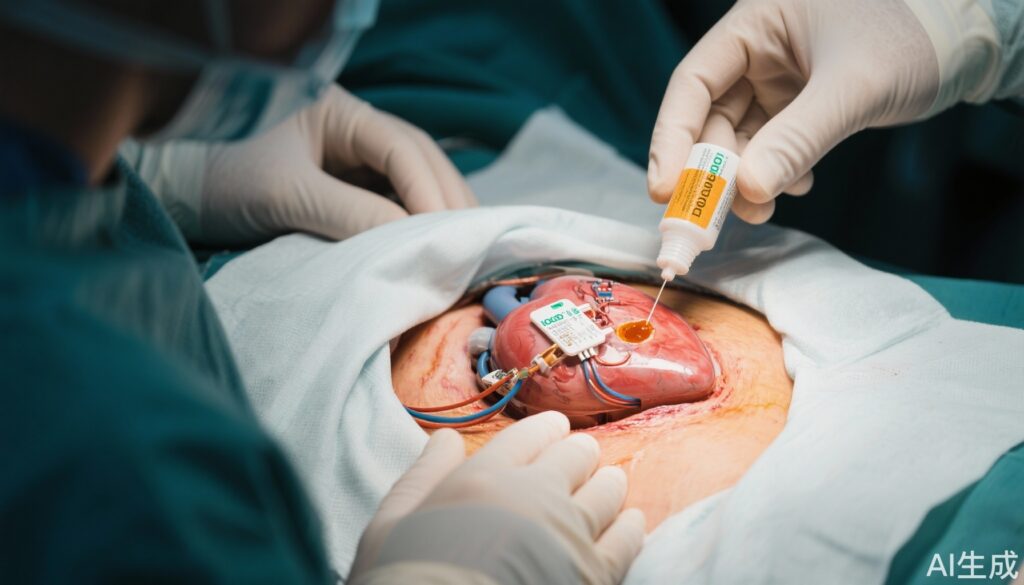
This prospective, randomized clinical trial was conducted at a single center between November 2020 and May 2024. The study enrolled 418 patients undergoing repeat procedures involving the same device pocket for CIEDs. Patients were randomized in a 1:1 ratio to receive either an intraoperative adhesive iodine-impregnated drape applied immediately at the start of the procedure or standard care without iodine drapes. To reduce bias, patients, swab-performing staff, and microbiologists evaluating cultures were blinded to group allocation, constituting a single-blinded design. The primary endpoint was the rate of positive pocket-swab cultures obtained at the end of the procedure, serving as a surrogate marker for surgical site contamination. Secondary endpoints included adjudicated CIED infections observed up to one year post-procedure.
Key Findings
Among the 418 randomized patients, 189 in the iodine drape group and 195 in the control group completed the study analysis. Both groups were well matched regarding age (mean ~73 years) and sex distribution (approximately 73% male). The key result was a significant reduction in pocket-swab culture positivity in the iodine drape group (10.1%) versus controls (20.5%), conferring a relative risk reduction of 50% (95% CI: 24%-75%; P = .005). This finding underlines the efficacy of iodine-impregnated drapes in decreasing microbial contamination during repeat CIED surgeries.
Adjudicated CIED infections within one year were reported in 4 of 195 patients (1.9%) in the no-drape group and none in the iodine-drape group, with statistical significance (P = .02). Although infections were more frequent in patients with positive swabs (3.4%) versus negative swabs (0.6%), this difference did not reach statistical significance (odds ratio 5.67, 95% CI: 0.78-41.04; P = .08), possibly reflecting limited event numbers.
No adverse events related to iodine drape use were reported, supporting its safety profile. These findings demonstrate that the simple addition of an iodine-impregnated drape reduces pocket contamination at surgery, translating into lower infection rates—a profound benefit in clinical practice.
Expert Commentary
This trial substantiates a mechanistically plausible and clinically relevant preventive strategy against repeat CIED infections. Bacterial inoculation at the surgical site is central to infection development. The antiseptic properties of iodine, combined with the adhesive barrier that iodine-impregnated drapes provide, likely reduce microbial transfer from skin flora and the environment into the pocket.
While the study’s single-center design may limit broad generalizability, the randomized, blinded methodology and well-matched cohorts strengthen internal validity. Future multi-center trials could confirm these findings and assess cost-effectiveness across diverse healthcare settings. There is also a need to determine whether iodine drapes confer benefit in initial CIED implants or other device surgeries prone to infection.
The observed association between positive swabs and infection highlights the predictive value of intraoperative cultures and potential to stratify infection risk for tailoring prophylaxis or early interventions.
Conclusion
The application of intraoperative iodine-impregnated adhesive drapes during repeat CIED implantation effectively halves pocket contamination rates and abolishes adjudicated device infection incidence over one year. This aligns well with current understanding of infection pathogenesis and resistance mechanisms, offering a straightforward, safe, and cost-effective measure to improve patient outcomes. Wider adoption could substantially decrease the clinical and economic burden of CIED infections, a growing problem with aging patient populations and increasing device-related procedures. Ongoing research should expand on these findings to optimize perioperative protocols for CIED and other implantable device surgeries.
References
Aydin A, Golian M, Klein A, Redpath C, Davis DR, Ramirez FD, Nair GM, Green MS, Sadek M, Nery PB, Hansom SP, Al Hinai G, Weng W, Berbenetz N, Thibert MJ, Salmeen Y, Martow E, Tan LW, Almidani G, Alshehri A, Alzahrani A, Alharbi F, Corrales-Medina V, Wells GA, Birnie DH. Iodinated Adhesive Drapes for Repeat Cardiac Implantable Device Implantation: A Randomized Clinical Trial. JAMA Cardiol. 2025 Aug 27:e252835. doi: 10.1001/jamacardio.2025.2835. Epub ahead of print. PMID: 40864462; PMCID: PMC12392146.
Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422-1429. doi:10.1056/NEJMra035415
Tarakji KG, Uslan DZ, Wilkoff BL. Update on Prevention and Management of Infection With Cardiac Implantable Electronic Devices. Cardiol Clin. 2015;33(1):119-132. doi:10.1016/j.ccl.2014.08.004
Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement From the American Heart Association. Circulation. 2010;121(3):458-477. doi:10.1161/CIRCULATIONAHA.109.192665