Highlight
– Personalized HD-tDCS significantly reduces depressive symptoms in moderate to severe depression compared to sham control.
– Effect size (-0.50) observed after 12 sessions is comparable to pharmacotherapy and psychotherapy but with faster onset.
– HD-tDCS is well-tolerated, causing only mild adverse effects.
– Exploratory findings suggest concurrent reduction in anxiety symptoms associated with active HD-tDCS treatment.
Study Background and Disease Burden
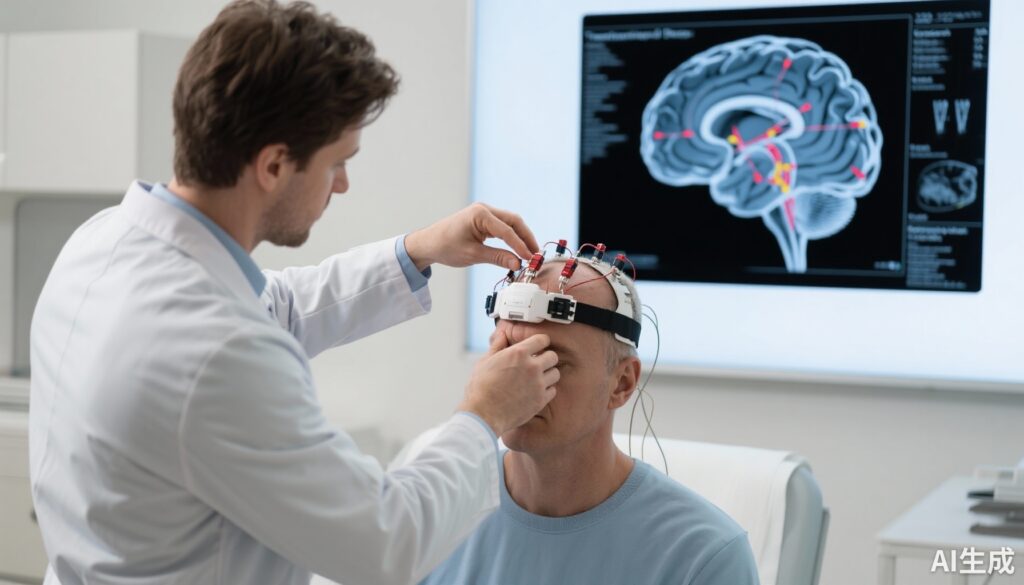
Major depressive disorder (MDD) is a prevalent and debilitating mental health condition contributing substantially to global disability and economic burden. Despite numerous antidepressant medications and psychotherapeutic interventions, a significant proportion of patients experience suboptimal response or delayed therapeutic onset. Conventional transcranial direct current stimulation (tDCS), a noninvasive brain stimulation modality that modulates cortical excitability, has shown antidepressant potential but with variable efficacy, partly due to imprecise stimulation targeting and limited focality. The advent of personalized high-definition tDCS (HD-tDCS), which leverages advanced neuroimaging and neuronavigation to tailor stimulation to individual neuroanatomy, offers a promising pathway to enhance therapeutic outcomes through more precise and focal cortical modulation. This randomized clinical trial addresses the unmet clinical need of developing more efficacious and tolerable rapid-onset treatments for moderate to severe depression.
Study Design
This double-blind, sham-controlled, parallel-group randomized clinical trial was conducted at the University of California, Los Angeles (UCLA) from December 1, 2020, to March 7, 2024. The study enrolled 71 adults aged 18 to 65 who met diagnostic criteria for a current major depressive episode, as assessed by the Mini International Neuropsychiatric Interview, and had moderate to severe depressive symptoms (Hamilton Depression Rating Scale [HAMD] scores >=14 but less than 24). Participants were either antidepressant treatment naive or maintained on a stable standard antidepressant regimen. Key exclusions included treatment-resistant depression, bipolar disorder, or schizophrenia.
Participants were randomized to receive either active or sham HD-tDCS therapy for 20 minutes daily over 12 consecutive working days. Personalized HD-tDCS montages were generated using individual structural magnetic resonance imaging (MRI) scans combined with frameless stereotaxic neuronavigation to optimize stimulation focality and precision targeting of depression-relevant cortical regions. Primary outcome assessment was change in HAMD score from pretreatment baseline to posttreatment. Secondary outcomes included tolerability, adverse events, and exploratory analysis of anxiety symptom changes.
Key Findings
Of the 71 randomized participants (40 active, 31 sham; mean age 34.3 years; 62% female), baseline characteristics were comparable between groups. The primary endpoint demonstrated a statistically significant difference in mean HAMD score reduction favoring active HD-tDCS by 2.2 points (group difference, -2.2 [SD 4.3]; P = .04), with a moderate effect size (Cohen d = -0.50; 95% CI, -0.99 to -0.01). Within-group analyses revealed significant reductions in depression severity post-intervention: mean decrease of 7.8 points in the active group versus 5.6 points in the sham group. This indicates that while sham stimulation also produced improvements—likely reflecting placebo effects or nonspecific factors—the active stimulation conferred additional clinically meaningful benefit.
HD-tDCS was well tolerated with mostly mild adverse effects, including transient scalp tingling or redness, and no serious adverse events reported. Importantly, exploratory analyses identified a significant active treatment-related improvement in the anxiety dimension component of the HAMD (group difference = -0.68; P = .049; Cohen d = -0.48), suggesting potential dual efficacy for comorbid anxiety symptoms.
Compared with effect sizes observed in pharmacotherapy, psychotherapy, and conventional tDCS, which typically manifest over longer treatment durations, the moderate effect size after just 12 sessions underscores the advantage of personalized HD-tDCS in delivering rapid antidepressant effects. The precise cortical targeting enabled by MRI-guided neuronavigation likely contributes to enhanced neuromodulatory efficiency.
Expert Commentary
This trial aligns with emerging evidence supporting neuromodulation techniques as adjunct or alternative treatments for depression, especially for populations where pharmacologic treatments are contraindicated or not well tolerated. The use of personalized neuroimaging-guided HD-tDCS represents an important methodological advancement compared with traditional tDCS protocols, which often utilize generic electrode placements that may not optimally engage relevant neural circuits.
However, several limitations warrant attention. The sample size, while adequate for detecting moderate effects, limits external generalizability. Exclusion of treatment-resistant depression restricts applicability to that challenging subgroup. The 4-week follow-up period precludes definitive conclusions about the durability of antidepressant effects. Future studies should incorporate longer-term follow-up, maintenance stimulation strategies, and comparisons with active pharmacologic or psychotherapeutic treatment arms for a comprehensive evaluation.
Mechanistically, the improved efficacy may stem from precise targeting of dorsolateral prefrontal cortex subregions implicated in mood regulation, as well as modulation of anxiety-related neural networks. Additional neurophysiological and biomarker studies could elucidate pathways mediating clinical response and guide individualized treatment optimization.
Conclusion
This rigorously conducted randomized clinical trial demonstrates that personalized HD-tDCS is a safe, well-tolerated, and effective nonpharmacologic intervention for moderate to severe depression, producing significant mood improvements with a rapid onset and moderate effect size. The technique’s ability to concurrently reduce anxiety symptoms further increases its clinical utility. These findings support further development of precision neuromodulation approaches and justify larger-scale trials to establish long-term efficacy, maintenance protocols, and integration into standard depression treatment paradigms. Personalized HD-tDCS may represent a valuable addition to the therapeutic armamentarium addressing the global burden of depression.
References
Jog MA, Norris V, Pfeiffer P, Taraku B, Kozikowski S, Schneider J, Boucher M, Iacoboni M, Woods R, Narr K. Personalized High-Definition Transcranial Direct Current Stimulation for the Treatment of Depression: A Randomized Clinical Trial. JAMA Netw Open. 2025 Sep 2;8(9):e2531189. doi:10.1001/jamanetworkopen.2025.31189. PMID: 40932717; PMCID: PMC12426800.
Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. Transcranial Direct Current Stimulation for the Acute Treatment of Major Depressive Episodes: Meta-analysis of Individual Patient Data. Br J Psychiatry. 2016;208(6):522-531.
Kuo MF, Paulus W, Nitsche MA. Therapeutic Effects of Non-invasive Brain Stimulation with Direct Currents (tDCS) in Neuropsychiatric Diseases. Neuroimage. 2014 Jan 15;85 Pt 3:948-960.
Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based Guidelines on the Therapeutic Use of Transcranial Direct Current Stimulation (tDCS). Clin Neurophysiol. 2017 Jan;128(1):56-92.