Highlight
• In patients with heart failure with preserved ejection fraction (HFpEF) and physiologic pacemakers, personalized accelerated pacing above the conventional 60 bpm standard demonstrated slower adverse event accrual in per-protocol analysis.
• The myPACE trial observational follow-up over 4 years showed that patients adhering to personalized pacing had fewer heart failure–related hospitalizations and improved event-free survival.
• Intention-to-treat analysis trended towards benefit but did not reach statistical significance, highlighting the need for further validation in larger trials.
Study Background and Disease Burden
Heart failure with preserved ejection fraction (HFpEF) accounts for approximately half of all heart failure cases and represents a growing public health challenge due to aging populations and comorbidities. Unlike heart failure with reduced ejection fraction (HFrEF), HFpEF currently lacks highly effective disease-modifying therapies, resulting in persistent morbidity and frequent hospitalizations. Patients with HFpEF often have chronotropic incompetence—an impaired ability to increase heart rate appropriately during physical activity—which can limit cardiac output and exercise tolerance.
Physiologic pacemakers, which preserve atrioventricular synchrony and normal conduction pathways, have been utilized in HFpEF patients for bradyarrhythmias. Typically, the pacing lower rate limit is set to 60 beats per minute (bpm), reflecting standard clinical practice. However, this fixed rate may be suboptimal for patients with chronotropic insufficiency. Accelerated personalized pacing tailored to an individual’s physiologic needs could potentially enhance cardiac performance and clinical outcomes by optimizing heart rate responsiveness.
Study Design
This article reports findings from an observational extension of the myPACE randomized clinical trial conducted at the University of Vermont Medical Center. The original randomized trial enrolled 100 patients with stage B or C HFpEF who had preexisting physiologic pacemakers. Enrollment occurred between June 2019 and December 2021, with follow-up extended to June 2023 (up to 4 years).
Participants were randomized to two groups:
1. Personalized accelerated pacing (myPACE group): Heart rate settings individualized above the standard 60 bpm based on clinical parameters.
2. Usual care: Fixed pacing rate set at 60 bpm.
The primary endpoint was cumulative adverse clinical event accrual—first and recurrent events of urgent heart failure or atrial fibrillation hospital visits, myocardial infarction, stroke, or death—during the open-label follow-up period. Secondary endpoints included event-free survival.
Analysis was performed on both intention-to-treat (ITT) and prespecified per-protocol (PP) populations. The PP analysis included patients who maintained their assigned heart rate pacing strategy through the entire follow-up.
Key Findings
Among the initial 100 patients (48 assigned to myPACE and 52 to usual care), the ITT analysis exhibited a favorable trend with myPACE but did not meet formal statistical significance:
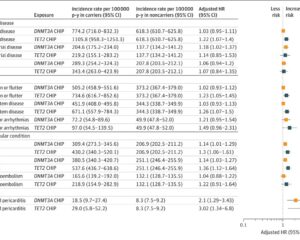
– Event accrual: 15 events in the myPACE group versus 33 events in usual care (Lin-Wei-Ying-Yang estimate [LWYY] 0.48, 95% CI 0.22-1.06, P=0.07).
– Event-free survival hazard ratio (HR) was 0.63 (95% CI, 0.31-1.29, P=0.20).
In the PP cohort (87 patients remaining adherent to assigned pacing: 39 myPACE, 48 usual care), stronger results were observed:
– Event accrual: 5 events in myPACE vs 31 in usual care (LWYY 0.16, 95% CI 0.04-0.67, P=0.01).
– Event-free survival HR was 0.30 (95% CI 0.11-0.80, P=0.02).
These clinical benefits were mainly driven by reduced heart failure-related adverse events, such as hospitalizations and urgent visits. The mean age of patients was 74 (±10) years, with 55% male participants. Safety profiles were comparable, with no new safety signals observed.
Expert Commentary
The myPACE trial provides compelling preliminary evidence that personalizing pacing rates above the conventional 60 bpm threshold can favorably influence clinical outcomes in HFpEF patients equipped with physiologic pacemakers. Given the pathophysiology of chronotropic incompetence in HFpEF, improved heart rate responsiveness through accelerated pacing is biologically plausible and mechanistically sound.
However, the lack of statistical significance in the ITT analysis suggests caution in generalizing these findings without confirmation in larger, multicenter randomized controlled trials. Adherence to pacing strategy emerged as a critical determinant of benefit, underscoring the importance of patient selection and management. Potential biases related to the open-label nature of the follow-up phase and single-center design should also be considered.
Current HF guidelines for HFpEF do not address pacing rate optimization, but this study suggests a novel therapeutic target warranting further exploration. Additional studies examining quality of life, functional capacity, and long-term mortality endpoints would be valuable.
Conclusion
Personalized accelerated physiologic pacing represents a promising intervention to reduce adverse clinical events and extend event-free survival in HFpEF patients with physiologic pacemakers. While the intention-to-treat results did not achieve statistical significance, per-protocol analyses showed significant clinical benefits primarily by reducing heart failure-related complications. These findings are hypothesis-generating and support further validation in larger, multicenter clinical trials to establish pacing rate personalization as a new standard of care in this challenging population.
References
Infeld M, Cyr J, Novelli AE, Rawlings R, Wahlberg K, Plante TB, de Lavallaz JDF, Habel N, Lustgarten DL, Meyer M. Clinical Outcomes With Personalized Accelerated Physiologic Pacing in Heart Failure With Preserved Ejection Fraction: Follow-up of the myPACE Trial. JAMA Cardiol. 2025 Aug 27:e252827. doi:10.1001/jamacardio.2025.2827. Epub ahead of print. PMID: 40864451; PMCID: PMC12392142.
Additional supporting literature:
– Shah SJ, Borlaug BA, Kitzman DW, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134(1):73-90.
– Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation. 2018;138(9):861-870.