Highlights
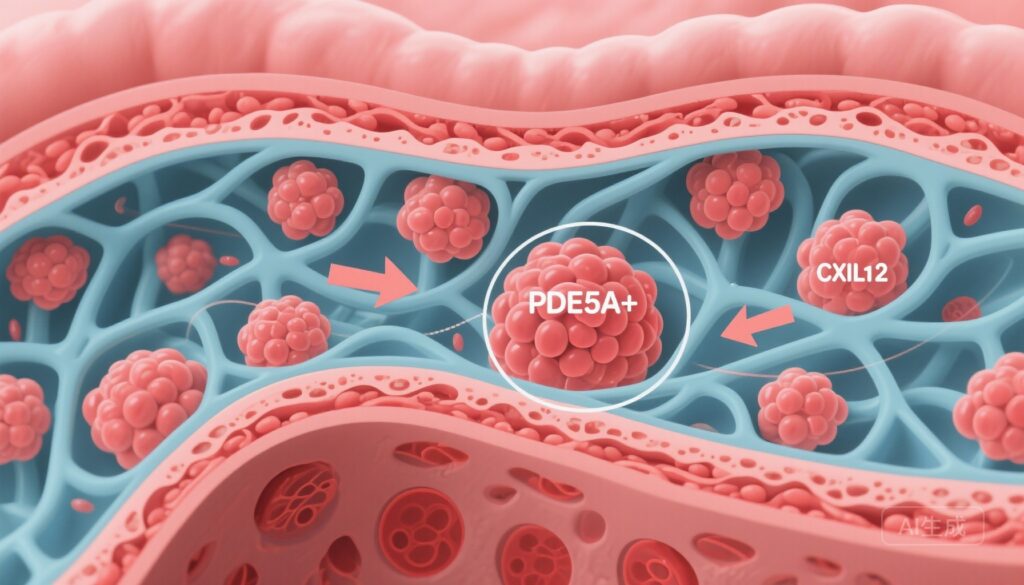
– A distinct PDE5A+ CAF subpopulation is associated with shorter overall survival and an immune‑excluded tumour microenvironment (TME) in gastric cancer (GC).
– PDE5A+ CAFs promote ECM remodelling and epithelial–mesenchymal transition (EMT) and activate PI3K/AKT/mTOR signalling to secrete CXCL12, recruiting CXCR4+ LAG3+ exhausted CD8 T cells.
– In preclinical models, pharmacologic inhibition of PDE5A with vardenafil combined with LAG3 blockade significantly improves antitumour immunity and reduces tumour growth, supporting translational evaluation.
Background and clinical context
Gastric cancer remains a major global cause of cancer mortality. Despite advances in surgery, chemotherapy and targeted agents, durable benefit from immune checkpoint inhibitors (ICIs) is limited to a subset of patients. A major determinant of ICI unresponsiveness is the tumour microenvironment (TME), where non‑malignant stromal cells — particularly cancer‑associated fibroblasts (CAFs) — establish physical and biochemical barriers that exclude cytotoxic T lymphocytes (CTLs) or skew immune cells toward dysfunctional or suppressive states. Identifying actionable CAF subsets that mediate immune suppression could open new combinatory treatment strategies to sensitize GC to immunotherapy.
Study design and methods
Wang and colleagues performed integrated single‑cell RNA sequencing (scRNA‑seq) on tumour samples from 24 patients with gastric cancer, together with spatial transcriptomics on formalin‑fixed paraffin‑embedded (FFPE) tissue sections from cases and controls. These multimodal data were used to map cellular compositions, define CAF subpopulations, and associate molecular signatures with clinical outcomes and spatial immune patterns.
Key experimental approaches included: identification of transcriptionally distinct CAF clusters; correlation of CAF signatures with overall survival and CD8+ T cell infiltration; in vitro functional assays assessing CAF effects on gastric cancer epithelial cells and matrix remodelling; mechanistic studies of signalling pathways by which CAFs secrete chemokines; and in vivo mouse models testing combination therapy with a PDE5 inhibitor (vardenafil) and LAG3 immune checkpoint blockade.
Key findings
1. Discovery of PDE5A+ CAFs and clinical associations
Using scRNA‑seq and spatial mapping, the investigators identified a reproducible CAF subpopulation characterized by high expression of phosphodiesterase type 5A (PDE5A). The PDE5A+ CAF signature correlated with worse overall survival in patients with GC and with histologic features of an immune‑excluded TME, including reduced infiltration of CD8+ cytotoxic T lymphocytes into tumour nests and enrichment of T cells at stromal margins.
2. ECM remodelling and promotion of EMT
PDE5A+ CAFs displayed upregulation of genes linked to extracellular matrix (ECM) deposition and remodelling. Functionally, coculture and conditioned‑media experiments showed that these CAFs promoted epithelial–mesenchymal transition (EMT) phenotypes in GC cells (e.g., increased vimentin, decreased E‑cadherin), which can promote invasion and may further contribute to immune exclusion by altering tissue architecture and stiffness.
3. PI3K/AKT/mTOR activation and CXCL12 secretion
Mechanistic interrogation revealed that PDE5A+ CAFs activate the PI3K/AKT/mTOR signalling cascade, leading to increased release of the chemokine CXCL12. CXCL12 is a potent chemoattractant for CXCR4‑expressing cells and has been implicated in exclusion of effector T cells and recruitment of suppressive or exhausted lymphocyte populations in several solid tumours.
4. Recruitment of exhausted LAG3+ CD8 T cells
Spatial and phenotypic analyses showed PDE5A+ CAF regions were enriched for CD8+ T cells expressing exhaustion markers, including LAG3 and features compatible with terminal exhaustion (TEX+). The data support a model in which CAF‑derived CXCL12 binds CXCR4 on CD8+ T cells, promoting their retention in stromal compartments and driving or maintaining an exhausted phenotype marked by LAG3 expression, thereby blunting effective antitumour responses.
5. Therapeutic combination: PDE5 inhibition plus LAG3 blockade
In murine models of gastric cancer, pharmacologic inhibition of PDE5A using vardenafil reduced CAF‑mediated signalling outputs and decreased CXCL12 levels. Monotherapy with either vardenafil or LAG3 blockade produced modest antitumour effects, but their combination synergistically increased intratumoural infiltration of functional CD8+ T cells, reduced exhausted T cell phenotypes, and suppressed tumour growth more effectively than either agent alone.
Mechanistic interpretation and biological plausibility
The proposed mechanism integrates stromal biology, chemokine‑driven leukocyte trafficking, and T cell dysfunction. PDE5A is a cyclic GMP (cGMP) phosphodiesterase; in CAFs its upregulation appears linked to downstream activation of PI3K/AKT/mTOR signalling — a pathway known to regulate protein synthesis, secretory phenotypes, and cell survival. By increasing CXCL12 secretion, PDE5A+ CAFs generate chemokine gradients that retain CXCR4+ lymphocytes in the stroma and prevent effective CTL engagement with malignant epithelial cells. Sustained exposure to such a suppressive stromal niche may drive or maintain expression of inhibitory receptors such as LAG3, consistent with the observed enrichment of LAG3+ exhausted CD8 T cells (TEX+ phenotype). Targeting both the stromal chemoattractant axis (by reducing CXCL12 production with PDE5 inhibition) and the checkpoint mediating functional exhaustion (LAG3 blockade) provides a rational two‑pronged approach to recondition the TME.
Translational and clinical implications
There are several attractive translational elements in this work: first, PDE5 inhibitors such as vardenafil are orally available, clinically approved agents with known pharmacokinetics and safety profiles, which could accelerate repurposing efforts. Second, LAG3 blockade is clinically actionable — the anti‑LAG3 antibody relatlimab has regulatory approval in melanoma in combination with anti‑PD‑1 — making combination strategies feasible.
Potential clinical strategies include testing vardenafil (or other PDE5 inhibitors) with LAG3 antibodies and/or PD‑1 axis inhibitors in biomarker‑selected GC patients whose tumours display a PDE5A+ CAF signature or spatial evidence of immune exclusion. Spatial transcriptomics or immunohistochemistry panels to detect PDE5A expression in CAFs could serve as companion diagnostics to enrich trials for patients most likely to benefit.
Limitations and expert commentary
While the study provides compelling multimodal evidence, several caveats warrant emphasis. The primary human discovery cohort comprised 24 patients — adequate for exploratory scRNA‑seq but limited for definitive clinical correlation; validation in larger, independent cohorts is required. Preclinical efficacy was demonstrated in mouse models, which may not fully recapitulate human stromal complexity, immune repertoire, or pharmacodynamics of PDE5 inhibitors. Systemic PDE5 inhibition may have pleiotropic effects on vascular tone, immune cells, and non‑CAF stromal elements; dose selection and toxicity monitoring will be important in human trials.
Mechanistic questions remain: the upstream regulators of PDE5A induction in CAFs, potential interactions with other CAF subtypes (including myofibroblastic versus inflammatory CAFs), and the extent to which CAF targeting alters other immunosuppressive cell populations (e.g., regulatory T cells, myeloid cells) need further study. Finally, combinatory approaches must be evaluated carefully for additive toxicities and for optimal scheduling (concurrent vs. sequential administration).
Next steps and research priorities
Immediate priorities include: validation of the PDE5A+ CAF signature and its prognostic/predictive value in larger GC cohorts with linked outcomes to ICIs; in situ validation using multiplex immunofluorescence or spatial transcriptomics across diverse geographic and molecular GC subtypes; dose‑finding and pharmacodynamic studies of vardenafil in tumour‑bearing hosts to define stromal modulation; and early‑phase clinical trials, ideally as biomarker‑selected studies combining PDE5 inhibitors with LAG3 and/or PD‑1 blockade.
Conclusion
The work by Wang et al. characterizes a previously unrecognized PDE5A+ CAF subset that shapes an immune‑excluded TME in gastric cancer via ECM remodelling and PI3K/AKT/mTOR‑dependent CXCL12 secretion, recruiting exhausted LAG3+ CD8 T cells. Preclinical data demonstrating synergy of vardenafil and LAG3 blockade offer a plausible, clinically actionable strategy to reverse stromal‑mediated immune suppression. If validated in larger cohorts and early clinical trials, PDE5A‑targeted approaches could become part of biomarker‑guided combination immunotherapy for gastric cancer.
References
1. Wang K, Xie CJ, Ding Z, et al. PDE5A+ cancer‑associated fibroblasts enhance immune suppression in gastric cancer. Gut. 2025 Oct 20:gutjnl-2025-335794. doi:10.1136/gutjnl-2025-335794. PMID: 41115748.
2. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–598.