Highlights
– GFH375, an oral KRAS G12D selective inhibitor, demonstrated an investigator-assessed objective response rate (ORR) of 40.7% and disease control rate (DCR) of 96.7% in previously treated KRAS G12D-mutant pancreatic ductal adenocarcinoma (PDAC) in a multicenter phase I/II study presented at ESMO 2025.
– Median progression-free survival (PFS) was 5.52 months (90% CI 4.27–7.20); median overall survival (OS) not reached at median follow-up 5.65 months; safety profile was manageable with grade ≥3 treatment-emergent adverse events (TEAEs) in 31.8% and no treatment-related deaths reported.
Background and unmet need
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most lethal malignancies. Despite modest improvements over decades, population-level 5-year survival is still low. The majority of patients present with unresectable or metastatic disease and rely on systemic therapies, where clinical benefit is limited and resistance emerges quickly.
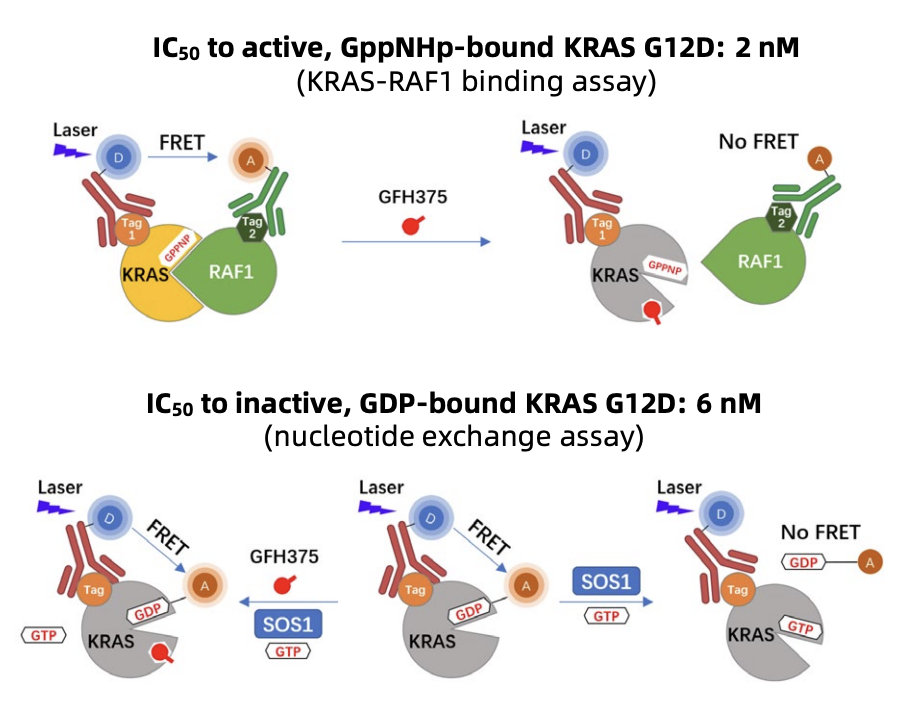
Mutations in the KRAS oncogene are the dominant molecular driver in PDAC; more than 90% of tumors harbor a KRAS mutation and KRAS G12D is a common subtype. Historically, KRAS has been considered a difficult drug target because of its biochemical properties and lack of accessible binding pockets. Recent advances in drug design and structure-guided approaches have produced selective KRAS inhibitors, first for G12C and now agents directed at other hotspot mutants such as G12D. Demonstrating meaningful clinical activity in KRAS G12D–mutant PDAC would represent a major therapeutic advance for this high‑need population.
Study design and methods
The data presented at ESMO 2025 come from a global, multicenter phase I/II study (NCT06500676) of GFH375, an oral, selective KRAS G12D inhibitor. The phase I portion included dose escalation (100 mg QD to 900 mg QD and 300 mg BID) and expansion; phase II enrolled several tumor-specific cohorts including PDAC, non‑small cell lung cancer, and colorectal cancer. The dataset reported at ESMO focuses on PDAC patients treated at the recommended phase II dose (RP2D) of 600 mg once daily (QD).
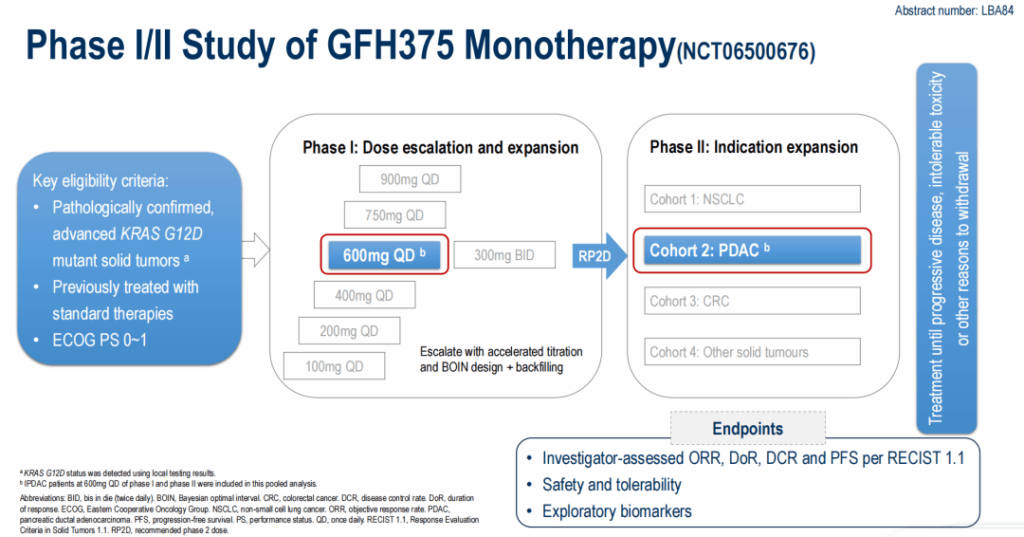
Key eligibility criteria included advanced or metastatic PDAC with a documented KRAS G12D mutation and prior systemic therapy (many patients had received multiple prior lines). Primary endpoints for the presented cohort were investigator-assessed ORR per RECIST v1.1, disease control rate (DCR), duration of response (DOR), PFS, safety and tolerability. Exploratory analyses included circulating tumor DNA (ctDNA) profiling and assessment of co‑occurring genomic alterations.
Patient population
As of data cut-off (27 Sep 2025), 66 patients with KRAS G12D mutant advanced PDAC received GFH375; 95.5% were stage IV at enrollment. Common metastatic sites at baseline were liver (78.8%), lung (28.8%) and peritoneum (28.8%). Prior therapy was substantial: 68.2% had received ≥2 prior lines and 33.3% had prior immune checkpoint inhibitors. At the time of analysis, 31 patients (47.0%) remained on treatment.
Key efficacy results
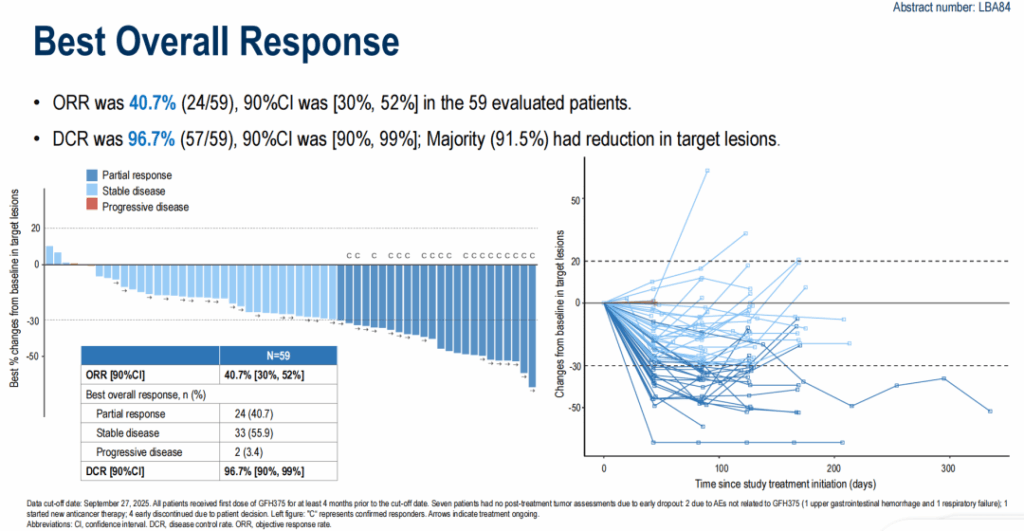
Among 59 evaluable patients, investigator-assessed responses were as follows:
- ORR: 40.7% (24/59) — all partial responses (PRs).
- Stable disease (SD): 55.9% (33/59).
- Progressive disease (PD): 3.4% (2/59).
- DCR: 96.7% (PR + SD).
- Percentage with any target lesion shrinkage: 91.5%.
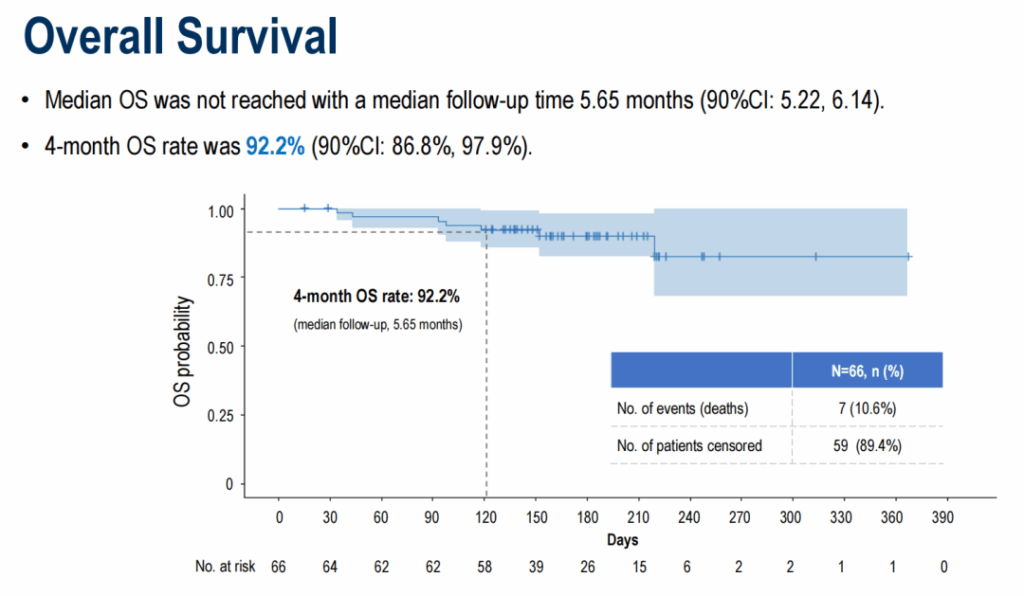
At a median follow-up of 5.65 months, median PFS was 5.52 months (90% CI 4.27–7.20). The 4‑month PFS rate was 78.2% (90% CI 69.8%–87.5%). Median OS had not been reached; the 4‑month OS rate was 92.2% (90% CI 86.8%–97.9%). Duration of response data were immature at the time of reporting.
Safety and tolerability
GFH375 treatment was associated with treatment-emergent adverse events in all patients (100%). Most treatment-related adverse events (TRAEs) were grade 1–2 and predominantly gastrointestinal or hematologic in nature. Grade ≥3 TEAEs/TRAE occurred in 31.8% (21 patients). No treatment-related deaths were reported. Investigators indicated most AEs were manageable with supportive care and dose modifications; the full safety dataset, including detailed AE types, time to onset, dose reductions, and discontinuations, will be important to review in full publications.
Biomarker analyses and subgroup observations
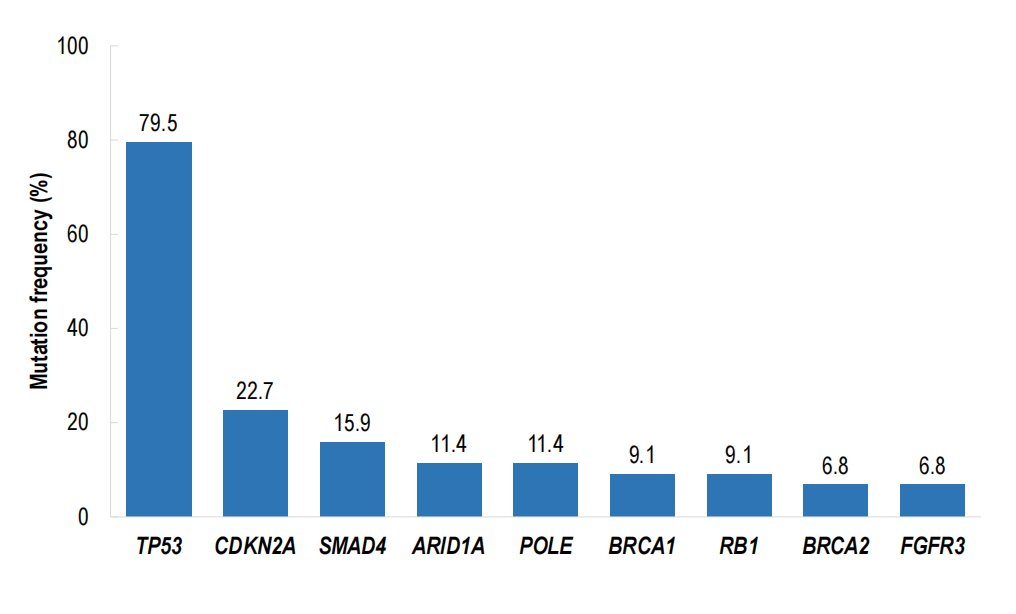
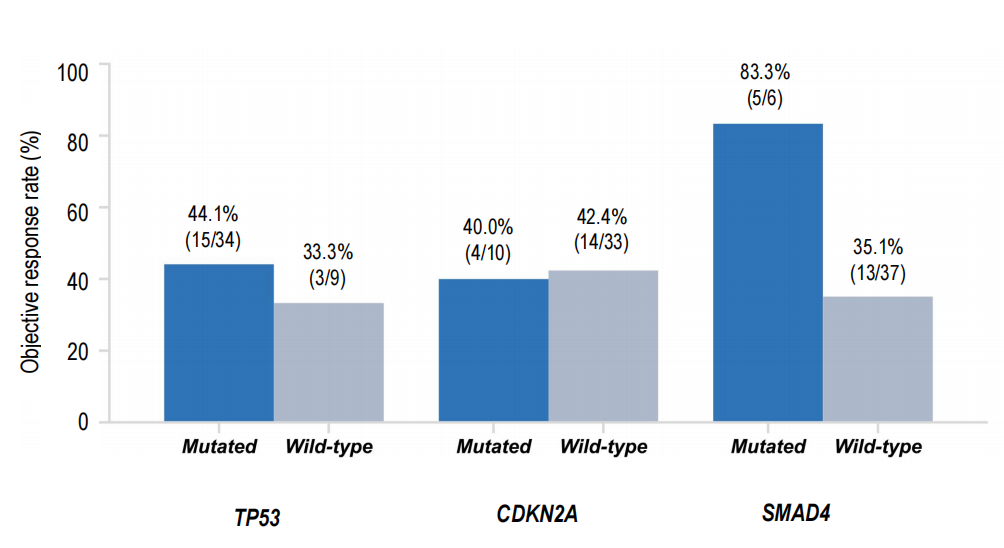
Exploratory ctDNA assessments and co‑mutation analyses were reported. Notably, responses were observed even in patients harboring co‑occurring PDAC-associated tumor suppressor alterations such as TP53 and SMAD4. A striking subgroup signal was observed for patients with SMAD4 co‑mutation where ORR reached 83.3% compared with 35.1% in SMAD4 wild‑type patients; this finding is hypothesis‑generating and requires cautious interpretation due to small subgroup sizes and potential selection biases.
Interpretation and clinical context
These results are compelling for several reasons. First, a single-agent ORR of 40.7% and DCR of 96.7% in a heavily pretreated PDAC population is substantially higher than typical response rates seen with later-line cytotoxic therapies historically used in this setting, where objective responses are uncommon. Second, evidence of activity across patients with adverse co‑mutational profiles suggests a potentially broad therapeutic window for KRAS G12D–driven disease.
Mechanistically, GFH375 is described as a non‑covalent inhibitor that can engage KRAS G12D in both its GDP‑bound (inactive) and GTP‑bound (active) states, blocking downstream effector signaling. If confirmed in translational studies, dual‑state engagement could help explain the magnitude and durability of responses observed in some patients compared with prior attempts to selectively inhibit KRAS variants.
Limitations and caveats
Important limitations include the single‑arm, nonrandomized design and relatively short median follow‑up at the time of reporting. Responses were investigator assessed; independent central review and mature survival endpoints will be needed to corroborate efficacy. The number of evaluable patients in some genomic subgroups (for example, SMAD4 co‑mutants) was small, so subgroup efficacy signals should be considered exploratory. Safety follow‑up is ongoing and longer exposure will be needed to define chronic toxicities and late events.
Next steps and ongoing development
Based on these data, a randomized phase III trial comparing GFH375 with chemotherapy in KRAS G12D–mutant metastatic PDAC was registered and publicly disclosed on November 11, 2025. The phase III design, endpoints, and eligibility criteria will determine whether the signal seen in phase I/II translates into a reproducible survival benefit and a potential new standard of care for this molecularly defined subgroup.
Expert commentary
GFH375 represents an important evolution in precision oncology for pancreatic cancer. KRAS has long been referred to as ‘undruggable,’ and the success of G12C inhibitors demonstrated that KRAS targeting is feasible. G12D is a different biochemical challenge and is more prevalent in PDAC; the reported clinical activity of GFH375 is therefore particularly noteworthy.
Clinicians should be cautiously optimistic. If phase III results confirm these findings, KRAS G12D testing will become essential for PDAC management and GFH375 may offer a biologically rational, targeted oral option for patients who have progressed on standard chemotherapy. Meanwhile, comprehensive biomarker studies, resistance mechanism mapping, and combination strategies (for example with chemotherapy, stromal modifiers, or immune therapies) should be prioritized to maximize benefit and overcome acquired resistance.
Conclusion
The ESMO 2025 presentation of GFH375 offers encouraging evidence that selective, orally available inhibition of KRAS G12D can produce substantial tumor responses and disease control in a heavily pretreated PDAC population, with a manageable safety profile. These results support continued development in randomized studies and underscore the importance of routine molecular profiling in PDAC to identify patients who may benefit from emerging targeted therapies.
Funding and trial registration
The phase I/II study discussed here is registered as NCT06500676. A randomized phase III study comparing GFH375 versus chemotherapy in KRAS G12D–mutant metastatic PDAC was registered and publicly posted on November 11, 2025. Funding sources were not detailed in the ESMO abstract; investigators reported support from the study sponsor. Full disclosures will be available in subsequent peer‑reviewed publications and regulatory filings.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49.
2. Wu Y, He S, Cao M, et al. Comparative analysis of cancer statistics in China and the United States in 2024. Chin Med J (Engl). 2024;137(24):3093-3100.
3. Zhou A, Li Z, Sun Y, et al. Efficacy and safety of GFH375 monotherapy in previously treated advanced KRAS G12D mutant pancreatic ductal adenocarcinoma (PDAC). Presented at: 2025 ESMO Congress; October 17-21, 2025; Berlin, Germany. Abstract: LBA84.
4. Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: progress and challenges. Nat Rev Drug Discov. 2014;13:828–51. (Review on historical challenges and progress in targeting KRAS)
Note to readers
This article summarizes aggregate results presented in an abstract at a major oncology meeting. Readers should consult the full peer‑reviewed manuscript, clinicaltrials.gov entries, and regulatory communications for complete data, updated survival outcomes, and detailed safety information when they become available.