Highlight
– This randomized clinical trial evaluates the effect of oral corticosteroids on unilateral vocal fold paralysis (VFP) after thyroid or parathyroid surgery.
– Corticosteroids given for 7 days postoperatively did not improve vocal cord mobility or voice quality compared to placebo.
– Vocal fold remobilization rates by day 7 and subsequent months were similar between corticosteroid and placebo groups.
– Speech therapy remains the cornerstone for managing postthyroidectomy VFP.
Study Background and Disease Burden
Unilateral vocal fold paralysis (VFP) is a notable complication occurring after thyroidectomy or related cervical surgeries, with incidence rates reported between 2-6%. While the majority of cases involve temporary dysfunction due to neuropraxia or inflammation affecting the recurrent laryngeal nerve, the clinical consequences of VFP range from hoarseness and voice fatigue to aspiration, significantly impacting patients’ quality of life and functional communication.
In situations where repeated laryngeal nerve transection is excluded, it is hypothesized that local inflammation, postoperative edema, or nerve bruising may contribute to the impaired mobility of the vocal fold. Despite this pathophysiological rationale, there remains no consensus on the optimal management strategy to accelerate recovery or improve voice outcomes in this patient population. Oral corticosteroids, given their potent anti-inflammatory effects, have been proposed as a potential therapeutic intervention to mitigate nerve edema and facilitate earlier functional recovery. However, robust clinical evidence supporting this approach is lacking.
Study Design
This prospective, randomized, double-blind placebo-controlled clinical trial was conducted at a tertiary referral center between September 2018 and May 2023. Patients undergoing partial or total thyroidectomy or parathyroidectomy for benign indications were enrolled. Key exclusion criteria included intolerance to corticosteroids, prior neck surgery or radiotherapy, surgeries performed for malignant tumors, and presence of multinodular intrathoracic goiters.
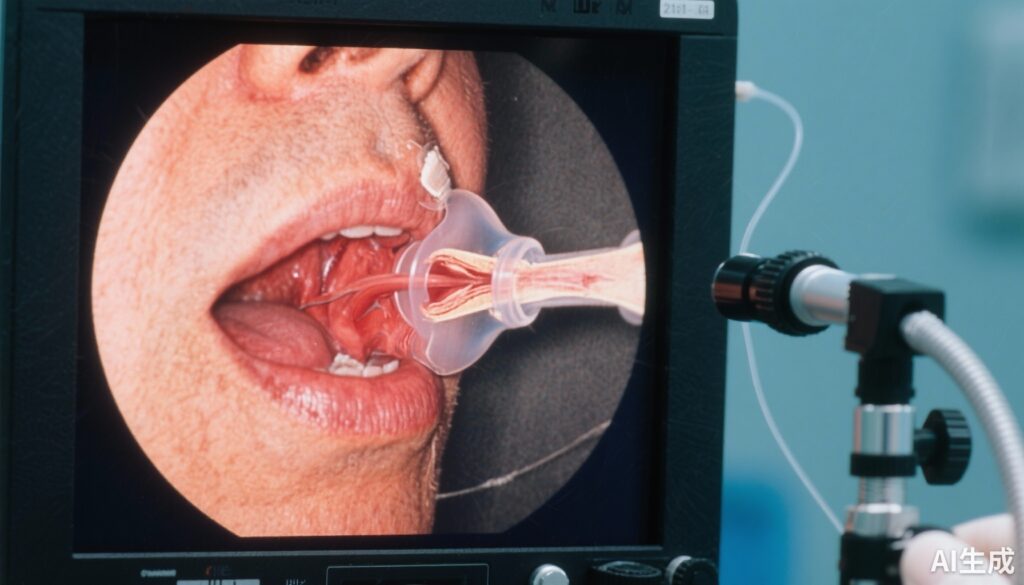
Baseline assessment involved comprehensive nasofibroscopic examination to evaluate vocal cord mobility and voice quality quantified using the standardized GRBAS scale, which grades the severity of voice impairment across five parameters: grade, roughness, breathiness, asthenia, and strain. Postoperatively, patients were systematically screened on day 1 for the presence of unilateral VFP.
Those diagnosed with unilateral VFP were randomized to receive either a 7-day course of oral corticosteroids or a matched placebo. Follow-up evaluations comprising nasofibroscopy and GRBAS scoring were performed at 7 days, 1 month, and 3 months post-surgery. Analyses adhered to the intention-to-treat principle.
Key Findings
Among 468 patients included, 26 (5.6%) developed unilateral VFP and were randomized—distributed as 14 patients in the corticosteroid group and 12 in the placebo group. Demographic characteristics were balanced: corticosteroid group had a median age of 68 years and placebo group 59 years, with a predominance of female patients (80.8%).
By postoperative day 7, vocal cord remobilization was observed in 42.8% (6/14) of the corticosteroid cohort and 41.6% (5/12) of the placebo cohort, yielding a negligible proportion difference of 1.2% (95% CI, -32.8% to 34.4%). No statistically significant or clinically meaningful differences were observed in voice quality as measured by GRBAS parameters at any assessment time point.
Treatment was well tolerated, with no serious corticosteroid-related adverse events reported during the study period. Longer-term follow-up at 1 and 3 months confirmed the absence of benefits from corticosteroids in promoting vocal fold mobility or improved phonatory function.
Expert Commentary
The findings of this rigorously conducted clinical trial challenge the previously hypothesized benefit of oral corticosteroids for postoperative unilateral VFP. While the rationale for corticosteroids to reduce nerve inflammation and enhance recovery remains biologically plausible, this trial indicates that a short 7-day course does not confer accelerated remobilization or better voice outcomes compared to placebo.
These results emphasize that the pathophysiology of postthyroidectomy VFP may involve complex nerve injury mechanisms beyond transient inflammation alone, including ischemia, fibrosis, or axonal damage that corticosteroids cannot readily reverse. Consequently, empiric corticosteroid use in this setting lacks evidence-based support.
Importantly, the trial reinforces the pivotal role of specialized speech therapy, which remains the mainstay for rehabilitation by promoting compensatory strategies and optimizing voice function during the recovery phase. Further research may explore other pharmacologic or regenerative interventions but must prioritize outcomes that incorporate patient-centered voice quality metrics and functional assessments.
Limitations of the study include the relatively small number of patients randomized due to the inherent incidence of postoperative VFP and potential variability in surgical and anesthetic techniques which may influence recovery. However, the double-blind design and standardized assessments strengthen the validity of conclusions.
Conclusion
In patients with unilateral vocal fold paralysis following thyroid or parathyroid surgery, oral corticosteroids administered for seven days postoperatively do not enhance vocal fold remobilization nor improve subjective and objective voice quality measures compared with placebo. Current evidence does not support routine corticosteroid therapy for postoperative VFP, and clinicians should prioritize prompt referral to voice therapy and multidisciplinary management.
This trial offers critical high-level evidence to inform clinical practice guidelines and underscores the necessity for continued research into targeted therapies for nerve recovery after thyroid surgery complications.
References
Carvajal-Alegria E, Girault N, Donatini G, Frasca D, Tonnerre D, Cosset T, Rullière A, Lebreton JP, Dufour X, Carsuzaa F. Oral Corticosteroids in Vocal Fold Paralysis After Thyroid Surgery: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg. 2025 Sep 1;151(9):874-880. doi: 10.1001/jamaoto.2025.2169. PMID: 40773211; PMCID: PMC12332756.
Gross ND, Altman KW, Roy S. Post-thyroidectomy unilateral vocal cord paralysis: Timing and success of vocal fold medialization injection. Laryngoscope. 2020;130(6):1421-1427. doi: 10.1002/lary.28297.
Sulica L. Vocal fold paralysis: Rationale and treatment approaches. Otolaryngol Clin North Am. 2021;54(1):121-135. doi: 10.1016/j.otc.2020.08.005.
Bitterlich N, Plinkert PK, Staar S, et al. Therapy of unilateral vocal fold paralysis: Current concepts and future perspectives. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2018;17:Doc07. doi: 10.3205/cto000155.