Highlights
– OB756, a novel selective JAK2 inhibitor, induced rapid platelet and leukocyte reductions and symptom improvement in hydroxyurea- or interferon-intolerant/resistant essential thrombocythemia (ET) patients in a phase II multicenter study.
– At week 24 the intention-to-treat complete hematologic response (CHR) rate was 22%; hematologic response (HR) rates rose over time to 65.6% at week 24 and 90% by week 48 in evaluable patients.
– Spleen volume decreased in most evaluable patients (median –34.96% at week 24), and JAK2 V617F allele burden declined in a majority of assessable patients; adverse events were generally grade 1–2, with anemia and infections being the most common toxicities.
Background and clinical need
Essential thrombocythemia (ET) is a chronic Philadelphia-negative myeloproliferative neoplasm characterized by sustained thrombocytosis, thrombohemorrhagic risk, and symptom burden from vasomotor phenomena, fatigue, and splenomegaly in a subgroup of patients. Standard cytoreductive therapy for high-risk ET commonly includes hydroxyurea (HU) or interferon-alpha (IFN). A subset of patients, however, are intolerant of or resistant to these agents and require alternative strategies. Mutations that activate the JAK-STAT pathway, most frequently JAK2 V617F, are central to ET pathophysiology and provide a rationale for JAK-targeted therapy.
Ruxolitinib, a JAK1/2 inhibitor, has been evaluated for ET in patients who are resistant or intolerant to HU, with heterogeneous efficacy and a safety profile that includes cytopenias and infections. There remains an unmet need for well-tolerated, effective agents that selectively target JAK2-driven disease biology, reduce symptom burden and spleen volume, and ideally lower mutant allele burden without excessive off-target toxicity.
Study design
The trial reported by Huang et al. is a phase II, open-label, multicenter study conducted across 12 centers in China, enrolling adult patients (≥18 years) with ET who were intolerant of or resistant to hydroxyurea or intolerant of interferon. Eligible patients had ECOG performance status ≤2 and were treated with oral OB756 given twice daily at either 16 mg (exploratory subgroup, n=5) or 20 mg (n=45) in 28‑day cycles until disease progression or intolerance.
The prespecified primary endpoint was complete hematologic response (CHR) at week 24, defined as platelet count ≤400×10^9/L, white blood cell (WBC) count <10×10^9/L, absence of disease-related symptoms, and normalization of spleen size by imaging. Secondary endpoints included hematologic response (HR) rates over time, safety and tolerability, molecular responses (change in JAK2 V617F allele burden), spleen volume reduction of ≥35% (SVR35) at week 24, and symptom control measured by MPN-SAF TSS (Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score).
Patient population and baseline characteristics
Between November 2020 and May 2024, 96 patients were screened and 50 were enrolled and treated. Median age was 59 years (range 18–81), 42% were male, and median time from ET diagnosis to enrollment was 866 days. Prior therapy intolerance or resistance included HU intolerance (44%), HU resistance (28%), and IFN intolerance (28%). The cohort was molecularly typical for ET: 70% JAK2 V617F–positive, 20% CALR, and 2% MPL. Baseline median platelet count was high at 780×10^9/L (range 298–1929), WBC 6.9×10^9/L, and hemoglobin 139 g/L.
Key efficacy results
OB756 produced rapid reductions in platelet and leukocyte counts while sparing hemoglobin. In the 20 mg twice-daily group the median platelet count decreased from 778×10^9/L at baseline to a nadir of 512×10^9/L within 4 weeks; the 16 mg twice-daily exploratory group showed a slower reduction by week 6. WBC fell from a median of 6.9×10^9/L to 5.7×10^9/L within two weeks. Hemoglobin remained stable throughout treatment.
By intention-to-treat (ITT) analysis, CHR at week 24 was 22% (11/50). When assessed among evaluable patients at specific time points, CHR rates were 21.7% (10/46) at week 12 and 23.7% (9/38) at week 24. Hematologic response rates in evaluable patients rose over time: 56.6% at week 12 (26/46), 65.6% at week 24 (25/38), 76% at week 36 (19/25), and 90% at week 48 (9/10).
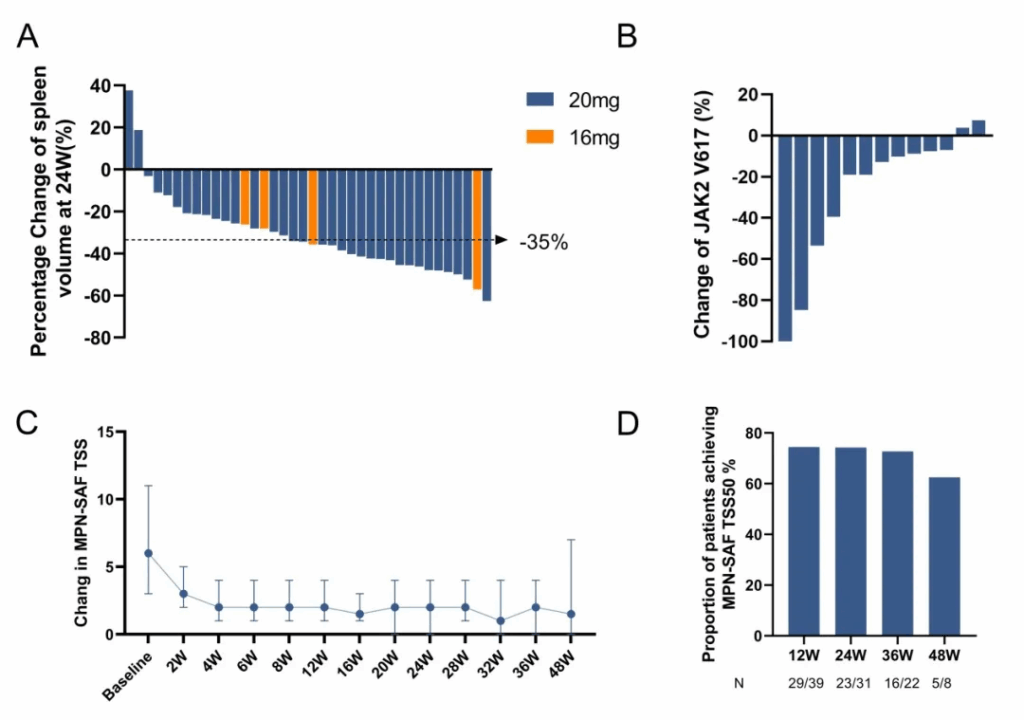
Spleen responses were notable within the subset of patients with evaluable imaging: at week 24, 94.7% (36/38) demonstrated any reduction in spleen volume with a median decrease of –34.96% (range –63% to +38%), and 50% (19/38) achieved SVR35 (≥35% reduction).
Molecularly, among 13 evaluable patients with baseline JAK2 V617F testing, 84.6% (11/13) showed reductions in allele burden after treatment; 61.5% (8/13) had ≥10% reductions. Symptom burden improved consistently: among 43 patients with baseline MPN-SAF TSS >0, the proportion achieving TSS50 (≥50% reduction) was 74.4% at week 12 and 74.2% at week 24, with sustained benefits seen at later time points in diminishing patient numbers.
Safety and tolerability
Overall, treatment-emergent adverse events (TEAEs) were predominantly grade 1–2. Hematologic toxicities included anemia in 52% (26/50) of patients, with grade ≥3 anemia in 10% (5/50); neutropenia occurred in 6% (3/50). Non-hematologic grade ≥3 adverse events were most commonly infections, observed in 16% (8/50), while 14 additional patients experienced grade 1–2 infections. Two patients (4%) experienced thrombotic events (four total events), all lacunar cerebral infarctions; investigators judged these unrelated to study drug. No cases of leukemic transformation or progression to overt myelofibrosis were recorded during the study period. There was one out-of-hospital death adjudicated as unrelated to OB756. In summary, the safety profile reported here is consistent with a tolerable toxicity spectrum dominated by modest cytopenias and infectious events; adverse events were described as clinically manageable.
Interpretation and clinical implications
The data indicate that OB756 achieves biologically and clinically meaningful effects in a population of ET patients who have limited alternatives because of intolerance or resistance to HU or IFN. Rapid platelet and WBC control, objective spleen volume reductions, symptomatic improvement (MPN-SAF TSS), and measurable decreases in JAK2 V617F allele burden support on‑target JAK2 inhibition and disease-modifying potential. Importantly, the CHR rate by ITT at week 24 (22%) appears modest, but HR rates in evaluable patients increased with time, and later time points showed higher response rates. This pattern suggests that some patients achieve clinically relevant control without meeting strict CHR criteria or that response matures with continued therapy. The relatively small sample size, open-label design, and the changing denominators for evaluable patients at different time points complicate straightforward interpretation and necessitate cautious comparison with existing agents. The favorable effect on JAK2 allele burden in most evaluable patients is encouraging, although the molecular sample size was small (n=13) and reductions were generally modest. Whether these molecular changes translate into long-term reductions in thrombotic risk, disease progression, or clonal evolution will require longer follow-up and larger, controlled trials.
Strengths and limitations
Strengths of the study include a multicenter design, enrollment of a real-world population of ET patients intolerant or resistant to first-line therapies, comprehensive hematologic, symptomatic, spleen, and molecular endpoints, and a reasonable treatment exposure (median ~11 months, with some patients treated >3 years). Limitations include the single-arm, open-label phase II design without a randomized comparator; small overall sample size and smaller evaluable subgroups for some secondary endpoints (notably molecular response); limited duration of follow-up for assessing long-term outcomes such as thrombosis, transformation to AML, or progression to myelofibrosis; and the absence of centrally adjudicated thrombosis assessment or blinded radiologic review in the provided summary. Safety signals—particularly infections and anemia—warrant ongoing surveillance in larger cohorts.
Next steps and research priorities
Key steps to advance OB756 toward clinical use should include randomized phase III trials comparing OB756 against current best alternatives in HU/IFN-intolerant or -resistant ET, standardized thrombosis monitoring and adjudication, larger molecular cohorts to characterize depth and durability of allele-burden responses, and longer follow-up to assess effect on progression, leukemic transformation, and survival. Dose optimization and exploration in other JAK2‑driven myeloproliferative neoplasms could be considered, along with translational studies to understand the drug’s selectivity profile and immunologic effects compared with JAK1/2 inhibitors.
Conclusion
In this phase II, multicenter study, OB756 demonstrated clinically meaningful platelet and leukocyte control, symptom relief, spleen volume reduction, and reductions in JAK2 V617F allele burden among ET patients intolerant of or resistant to hydroxyurea or intolerant of interferon. The safety profile was manageable, with predominantly low‑grade adverse events though infections and anemia were notable. These results support continued development of OB756 and justify randomized trials to determine its comparative efficacy, long-term safety, and impact on thrombotic risk and disease progression.
Funding and trial registration
Funding sources and clinical trial registration number were not provided in the summary available for this analysis. Readers should consult the full publication for detailed disclosures and trial identifiers.
Reference
Huang J, Li R, Gong T, Guo P, Shou L, Zhou H, Jiao Z, Zhang F, Liu Q, Zhao L, Wu G, Chen L, Zhou Y, Zhang Y, Jin J. Efficacy and safety of OB756 (a novel selective JAK2 inhibitor) for essential thrombocythemia in patients intolerant of or resistant to hydroxyurea or intolerant of interferon: A phase 2, open-label, multicenter study. Cancer. 2025 Oct 15;131(20):e70101. doi: 10.1002/cncr.70101 IF: 5.1 Q1 . PMID: 41060136 .