Highlight
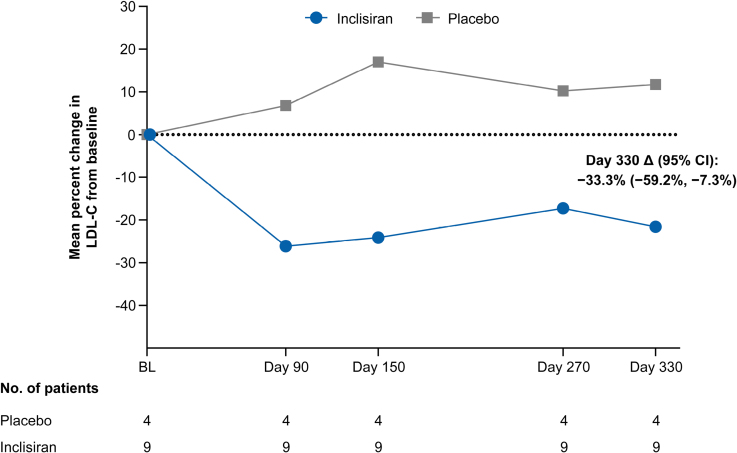
– Inclisiran achieved a placebo-adjusted LDL-C reduction of 33.3% over one year in adolescents with genetically confirmed HoFH.
– The therapy was well tolerated with no serious adverse events, supporting its safety profile in this young population.
– Significant reductions were observed in PCSK9 as well as apolipoprotein B and non-HDL cholesterol, reinforcing the biochemical efficacy.
– These findings suggest inclisiran as a promising adjunctive treatment for pediatric HoFH patients retaining some LDL receptor activity.
Study Background
Homozygous familial hypercholesterolemia (HoFH) is a rare inherited disorder marked by extremely elevated low-density lipoprotein cholesterol (LDL-C) from birth. This condition predisposes patients to rapid and severe atherosclerotic cardiovascular disease with onset often in childhood or adolescence. Despite statins and other lipid-lowering therapies, many affected individuals fail to achieve optimal LDL-C targets, underscoring a significant unmet need for more effective therapeutic options. This need is particularly urgent in pediatric patients, in whom early intervention can substantially modify long-term cardiovascular risk.
Inclisiran is a small interfering RNA (siRNA) that targets hepatic PCSK9 messenger RNA, effectively reducing circulating PCSK9 protein levels. PCSK9 plays a critical role in LDL receptor degradation; lowering PCSK9 enhances LDL receptor availability and LDL-C clearance. Inclisiran has demonstrated robust LDL-C lowering and good tolerability in adults with hyperlipidemia, but clinical data in pediatric populations—especially those with HoFH—were lacking prior to the ORION-13 study.
Study Design
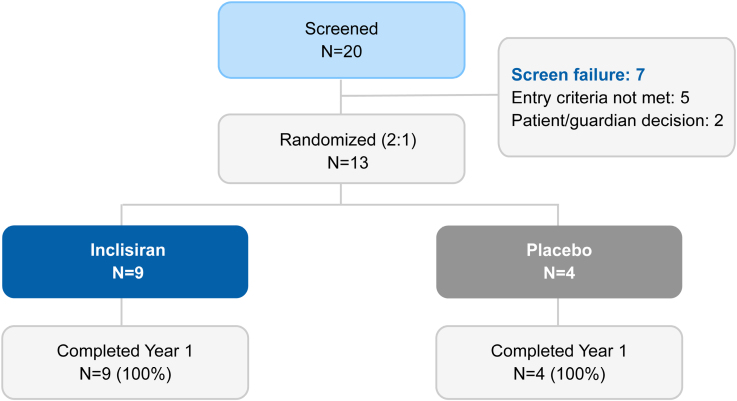
ORION-13 is a phase 3, multicenter, randomized, double-blind, placebo-controlled trial designed specifically to evaluate the efficacy and safety of inclisiran in adolescents aged 12 to younger than 18 years with genetically confirmed HoFH. This study excluded patients with LDL receptor (LDLR) null/null genotypes due to the anticipated minimal LDL receptor activity limiting treatment response.
A total of 13 patients with baseline LDL-C levels exceeding 130 mg/dL despite maximally tolerated statin therapy (with or without additional lipid-lowering agents) were enrolled. They were randomized in a 2:1 ratio to receive either 300 mg inclisiran sodium or placebo administered subcutaneously on days 1, 90, and 270. The primary endpoint was the mean percentage change in LDL-C from baseline to day 330.
Key Findings
The study population had a mean age of 14.8 years and notably elevated baseline LDL-C averaging 272 mg/dL. At day 330, the placebo-adjusted mean percentage reduction in LDL-C with inclisiran was 33.3% (95% confidence interval [CI], -59.2% to -7.3%), a clinically meaningful decrease given the refractory nature of HoFH.
Efficacy analyses showed that 66.7% (6/9) of patients receiving inclisiran achieved more than a 15% reduction in LDL-C compared to 25% (1/4) in the placebo group. Furthermore, 55.6% (5/9) of inclisiran-treated patients achieved LDL-C reductions greater than 20%, whereas none in the placebo arm reached this threshold.
Concomitant biomarker assessments revealed a robust decrease in PCSK9 levels by 60.2% (95% CI, -79.8% to -40.7%) relative to placebo, confirming the mechanism of action. This was paralleled by reductions in apolipoprotein B (-23.0%), non-high-density lipoprotein cholesterol (-32.7%), and total cholesterol (-27.8%).
Importantly, no serious adverse events, treatment discontinuations due to side effects, or deaths occurred during the study. The safety profile aligned with known data on inclisiran in adults, with no new concerns identified in this adolescent cohort.
Expert Commentary
The ORION-13 trial represents a landmark investigation of inclisiran in adolescents with HoFH, a group with few approved pharmacotherapies proven effective. The significant LDL-C reductions observed are promising, particularly given the challenges intrinsic to HoFH management and limited residual LDL receptor activity in included patients. These data provide mechanistic and clinical support for inclisiran’s role as an adjunct in this population.
However, some limitations exist, chiefly the small sample size intrinsic to this rare disease and exclusion of LDLR null/null genotypes. Longer-term follow-up will be essential to assess sustained efficacy and cardiovascular outcomes. Additionally, whether inclisiran’s twice-yearly dosing translates to improved adherence compared to existing therapies warrants further investigation.
Current guidelines endorse aggressive LDL-C lowering in HoFH but acknowledge the paucity of pediatric data for many agents. Inclisiran’s favorable safety profile and convenient dosing could address these gaps, pending broader confirmatory studies.
Conclusion
This 1-year, randomized, placebo-controlled study confirms that inclisiran effectively lowers LDL-C by approximately one-third and is well tolerated in adolescents with genetically confirmed HoFH possessing residual LDL receptor activity. These results mark an important advancement in pediatric lipid management, expanding therapeutic options for a population at extreme cardiovascular risk. Future research should focus on long-term benefits, cardiovascular event reduction, and inclusion of broader HoFH genotypes.
Inclisiran has the potential to become a valuable component of combination treatment strategies to achieve LDL-C targets more consistently in adolescents with HoFH, ultimately improving clinical outcomes.
References
1. Wiegman A, Peterson AL, Hegele RA, Bruckert E, Schweizer A, Lesogor A, Wang Y, Defesche J. Efficacy and Safety of Inclisiran in Adolescents With Genetically Confirmed Homozygous Familial Hypercholesterolemia: Results From the Double-Blind, Placebo-Controlled Part of the ORION-13 Randomized Trial. Circulation. 2025 Jun 24;151(25):1758-1766. doi: 10.1161/CIRCULATIONAHA.124.073233 IF: 38.6 Q1 . Epub 2025 May 20. PMID: 40391436 IF: 38.6 Q1 ; PMCID: PMC12180692 IF: 38.6 Q1 .
2. Cuchel M, Bruckert E, Ginsberg HN, Raal FJ. Homozygous Familial Hypercholesterolemia: New Insights and Treatments. Curr Atheroscler Rep. 2020;22(5):24.
3. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N Engl J Med. 2020 Jul 2;383(7):610-619.