Introduction
Biliary tract cancer (BTC) encompasses a heterogeneous group of malignancies including intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA), and gallbladder cancer (GB). These malignancies, rare and aggressive, represent a formidable clinical challenge given their silent progression, late diagnosis, and limited curative options—with only about 20% of patients eligible for surgical resection. The incidence of BTC varies globally and is rising, notably with increased mortality from iCCA in Western countries. Current standard first-line chemotherapy yields modest survival outcomes, with median overall survival (OS) around one year in stage IV disease. Recently, immune-checkpoint inhibitors (ICIs) combined with chemotherapy have emerged as a new standard, improving survival and sparking widespread research into enhancing therapeutic strategies across disease stages and molecular subsets.
The Impact of Tumor Immune Heterogeneity on Therapeutic Management
BTCs are characterized by a dense, desmoplastic stroma, rich in cancer-associated fibroblasts, contributing to a complex and immunosuppressive tumor microenvironment (TME). The immune cell infiltrate varies significantly across tumors, influencing prognosis and therapy response. Adaptive immune cells like CD4+ and CD8+ T lymphocytes correlate with favorable outcomes, whereas myeloid-derived suppressor cells and neutrophils typically portend poorer prognosis.
Large molecular studies classify BTC into immune subtypes, such as the inflammatory l2 and immunosuppressive l4 mesenchymal classes, the latter marked by stromal barriers inhibiting immune infiltration. Intratumoral heterogeneity, including variable immune cell states and plasticity, correlates with tumor aggressiveness and poorer clinical outcomes. These findings underscore challenges in selecting patients for ICIs and the limitations of current biomarkers such as programmed death-ligand 1 (PD-L1) expression, microsatellite instability (MSI), and tumor mutational burden (TMB). For example, clinical trials have demonstrated inconsistent correlation between PD-L1 status or TMB and treatment response in BTC, highlighting the need for more refined biomarkers.
Emerging liquid biopsy approaches like circulating tumor DNA (ctDNA) profiling hold promise for dynamic and noninvasive biomarker assessment.
Immune-Checkpoint Inhibitors in Advanced BTC
Monotherapy and Dual Immunotherapy
Single-agent PD-1 blockade (e.g., pembrolizumab or nivolumab) in BTC has demonstrated modest overall response rates (ORRs) around 6–13% in unselected or PD-L1-positive patients, with reliable biomarkers for patient selection still lacking. Dual checkpoint blockade combining anti-PD-1 and anti-CTLA-4 antibodies (nivolumab plus ipilimumab) has yielded limited improvements, with an ORR of 23% observed in select patients, primarily those with iCCA or GB carcinoma, irrespective of microsatellite status.
Combination of ICIs and Chemotherapy
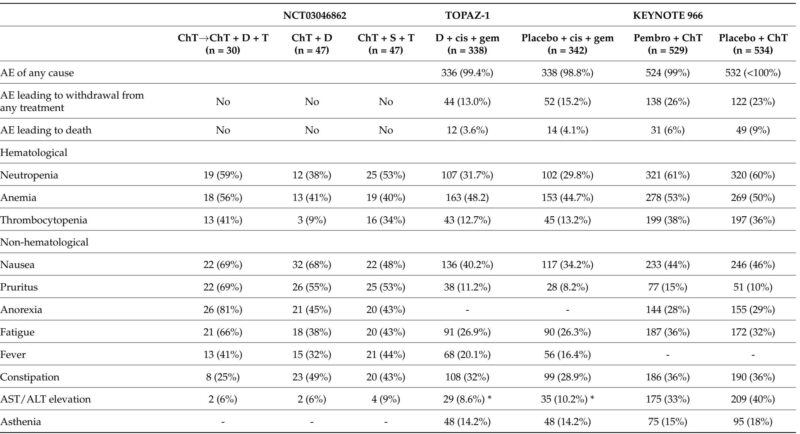
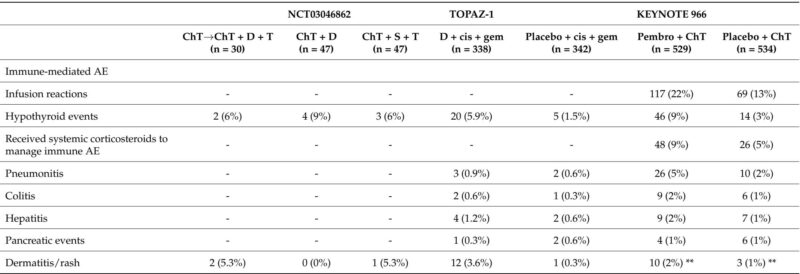
The most notable advances derive from combining ICIs with chemotherapy, elevating outcomes over chemotherapy alone. The Phase III TOPAZ-1 trial demonstrated that durvalumab plus cisplatin–gemcitabine significantly extended median OS to 12.8 months compared with 11.5 months for chemotherapy alone, with improved progression-free survival (PFS) and ORR. Importantly, durvalumab was well tolerated without a marked increase in toxicity. These findings were corroborated by the KEYNOTE-966 trial, where pembrolizumab combined with cisplatin–gemcitabine similarly improved survival compared to chemotherapy alone.
Predictive biomarkers remain under evaluation; early data suggest that conventional markers such as PD-L1 expression may have limited predictive power. Interestingly, recent analyses indicate potential associations between long-term survival and mutations in BRCA1/2, KRAS, and IDH1, warranting further validation.
Integration with Targeted Therapy
Molecularly targeted agents approved for BTC include inhibitors of IDH1 mutations (which occur in ~18-20% of iCCA) and FGFR2 fusions (~13-14%), predominantly in intrahepatic subtypes. Trials investigating combinations of ICIs with these targeted therapies (e.g., nivolumab with ivosidenib or pembrolizumab with pemigatinib) are ongoing, aiming to enhance efficacy and overcome resistance.
Moreover, combinations with MEK inhibitors and HER2-targeted therapies are being explored with varying results. MEK inhibition may modify the immune microenvironment to favor immunotherapy benefit, although clinical benefits observed thus far are modest. HER2 amplification occurs primarily in gallbladder and extrahepatic cholangiocarcinoma; antibody-based combinations incorporating pembrolizumab and trastuzumab are under investigation following success in other gastrointestinal tumors.
Poly (ADP-ribose) polymerase inhibitors (PARPi) combined with ICIs have shown limited efficacy in BTC to date, but ongoing trials continue exploring potential synergy, especially in homologous recombination deficiency contexts.
Combination with Antiangiogenic Agents
Tumor angiogenesis mediated by vascular endothelial growth factor (VEGF) contributes to an immunosuppressive TME and may impair ICI efficacy. Trials combining ICIs with antiangiogenic agents such as bevacizumab, lenvatinib, or regorafenib have demonstrated encouraging response rates and survival benefits, albeit with heterogeneous results. The LEAP-005 study and others show manageable toxicity and modest survival improvements, and combination therapies incorporating chemotherapy, ICIs, and antiangiogenics further improve outcomes in selected patient populations.
ICIs in Localized and Locally Advanced BTC
Surgical resection remains the sole curative option, but the majority of patients present with unresectable or advanced disease. Postoperative recurrence rates are high (50-70%), highlighting the unmet need for effective adjuvant strategies.
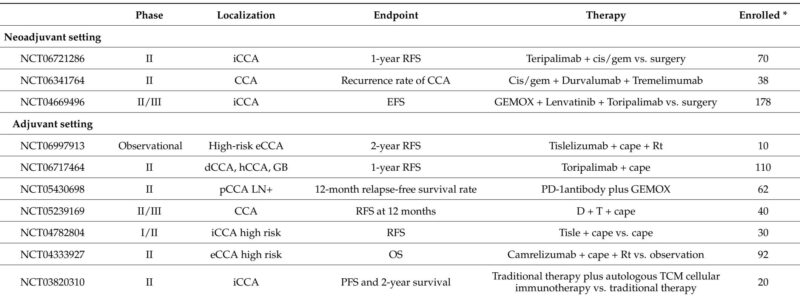
Adjuvant Immunotherapy
Capecitabine remains the standard adjuvant therapy based on the BILCAP study, though statistical significance was marginal. Recent trials are evaluating the addition of ICIs, alone or in combination with chemotherapy (e.g., durvalumab plus tremelimumab), to reduce relapse risk. Other trials explore combinations with radiotherapy and novel agents such as anti-PD-1/TIGIT bispecific antibodies. These studies aim to improve relapse-free and overall survival post-resection.
Neoadjuvant Immunotherapy
High rates of unresectable or locally advanced disease have stimulated interest in neoadjuvant strategies. Early-phase trials combining ICIs with chemotherapy or targeted therapies demonstrate promising objective response rates and improved resectability, supporting further investigation. Recent randomized studies (e.g., DEBATE trial) showed increased surgical exploration and R0 resection rates with durvalumab plus chemotherapy versus chemotherapy alone.
Future Directions
Beyond ICIs, novel immunotherapeutic approaches under development include bispecific antibodies (e.g., bintrafusp alfa targeting PD-L1 and TGF-β), CD40 agonists to activate antigen-presenting cells, cancer vaccines targeting antigens such as WT-1 and MUC1, and adoptive cell therapies including chimeric antigen receptor (CAR) T-cell therapies directed at mesothelin, HER2, and CEA.
Phase I-II clinical trials exploring these modalities are ongoing. Enhancing vaccine efficacy through adjuvants and nanotechnology, optimizing CAR T-cell targets, and combining immunotherapies with targeted agents hold promise but require robust validation.
Due to the complex tumor and immune heterogeneity of BTC, integrating comprehensive molecular profiling to guide therapy and developing dynamic biomarkers are essential steps moving forward.
Conclusion
Immune-checkpoint inhibitors have reshaped first-line treatment paradigms in metastatic BTC, particularly in combination with chemotherapy, reflecting moderate but meaningful survival benefits with manageable toxicity profiles. Expanding their role into adjuvant and neoadjuvant settings is an active area of investigation. The heterogeneity of BTC and its immunosuppressive microenvironment pose challenges to therapy and biomarker development.
Future advances will depend on refined patient selection through genomic and immune profiling, integration of multimodal therapies, and continued exploration of novel immunotherapeutic modalities. Collaborative efforts to unravel and target the interplay between tumor genomics, immune contexture, and the stromal environment will be critical to improving outcomes in this challenging malignancy.
Reference
Ceniceros L, Torre M, Landa Magdalena A, Sangro P, Argemí J, D’Avola D, Sangro B, Ponz-Sarvisé M. Immune-Checkpoint Inhibitors for Biliary Tract Cancer: Who Benefits and What Is Next? Cancers (Basel). 2025 Aug 28;17(17):2811. doi: 10.3390/cancers17172811 IF: 4.4 Q2 . PMID: 40940908 IF: 4.4 Q2 ; PMCID: PMC12427405 IF: 4.4 Q2 .