Highlight
- IBI311, an IGF-1R inhibitor analogous to teprotumumab, demonstrated an 85.8% proptosis response rate at 24 weeks in Chinese patients with active moderate to severe thyroid eye disease (TED), compared to 3.8% in placebo.
- The trial showed significant reductions in clinical activity score (CAS) and proptosis, confirming both anti-inflammatory and anatomical benefit in active TED.
- IBI311 exhibited a favorable safety profile with only mild to moderate adverse events and no serious adverse events reported.
Study Background
Thyroid eye disease (TED) is an autoimmune inflammatory disorder primarily affecting the orbit and periorbital tissues, often associated with Graves’ disease. It manifests clinically as proptosis, eyelid retraction, diplopia, and inflammation, which can lead to significant disfigurement, visual impairment, and diminished quality of life. TED has recognized phenotypic variability across races, impacting disease severity and response to therapies. Despite advances, treatment options remain limited, particularly for active moderate to severe TED.
Recent advances identified the insulin-like growth factor 1 receptor (IGF-1R) signaling pathway as central in TED pathogenesis. Teprotumumab, a human monoclonal antibody targeting IGF-1R, received FDA approval in 2020 based on pivotal trials largely in non-Asian populations. However, real-world access to such therapy in Asia is limited, and data on efficacy and safety in Asian, particularly Chinese, populations are scarce. IBI311 is an IGF-1R inhibitor with an identical amino acid sequence to teprotumumab but differs in dosage form, developed for use in Chinese patients.
Study Design
RESTORE-1 was a multicenter, randomized, double-masked, placebo-controlled phase 3 clinical trial conducted from May to December 2023 across 20 tertiary hospitals in China. Eighty-two participants with active moderate to severe TED (defined by clinical activity score [CAS] ≥3) were enrolled. Key exclusion criteria included TED onset greater than 270 days, sight-threatening TED, and prior steroid pulse therapy, radiotherapy, or orbital surgery.
Participants were randomized in a 2:1 ratio to receive intravenous infusions of IBI311 or placebo every three weeks over 21 weeks, with assessment continued through week 24. The primary endpoint was the proportion of participants achieving a proptosis reduction ≥2 mm in the study eye at week 24.
Secondary outcomes included combined response (proptosis and CAS reductions), CAS improvements, diplopia response, and safety assessments focused on known adverse events related to IGF-1R inhibition.
Key Findings
Among 82 randomized participants (mean age 39.6 years; 68.3% female), 54 received IBI311 and 28 received placebo. Results at week 24 were striking:
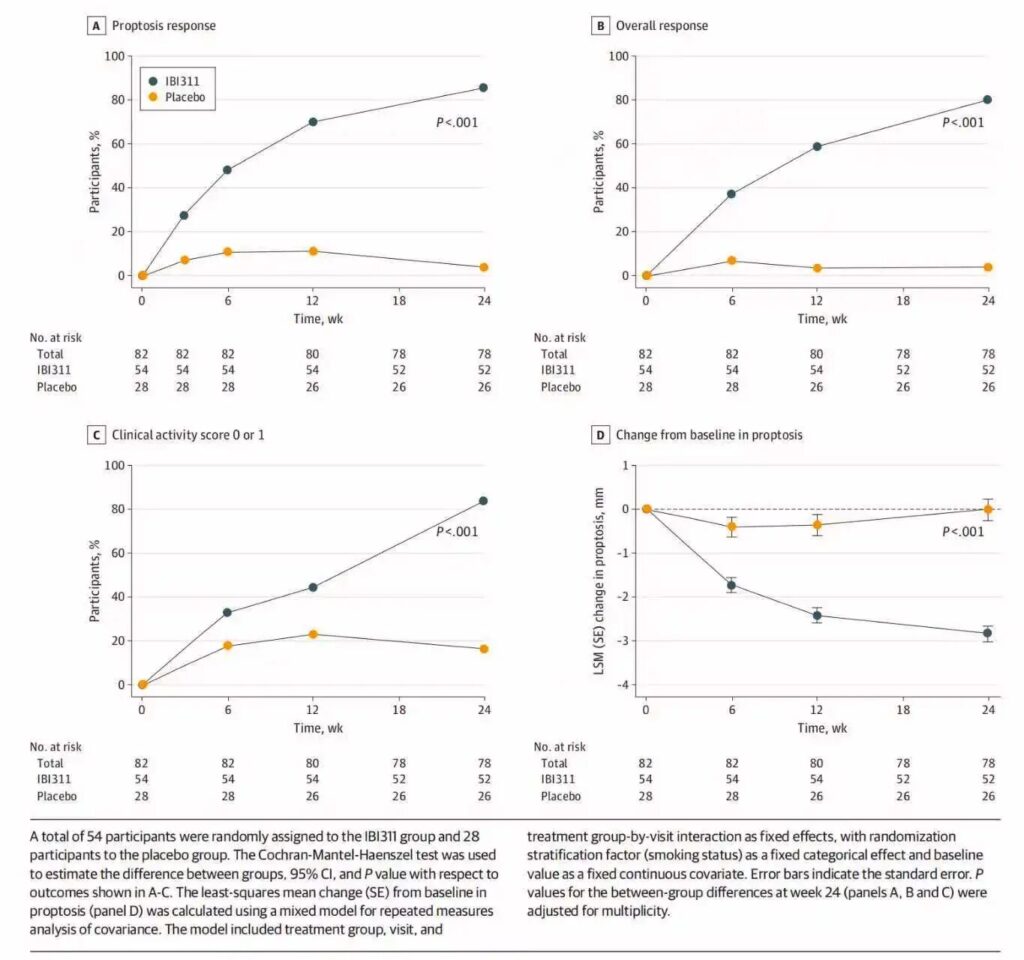
- Proptosis response: 85.8% (45/52) in the IBI311 group versus 3.8% (1/26) in the placebo group (difference 81.9 percentage points; 95% CI, 69.8 to 93.9; P < .001).
- Overall response: Combined proptosis reduction ≥2 mm and CAS reduction ≥2 occurred in 80.2% of IBI311 recipients versus 3.6% in placebo (difference 76.3 percentage points; 95% CI, 63.3 to 89.4).
- CAS improvement: Achieving CAS scores of 0 or 1 was attained in 83.5% versus 16.6% (difference 67.1 percentage points; 95% CI, 49.4 to 84.8).
- Proptosis magnitude: Least-squares mean change was -2.85 mm (SE 0.18) with IBI311 versus -0.02 mm (SE 0.24) with placebo (difference -2.83 mm; 95% CI, -3.39 to -2.27; all P < .001).
- Diplopia response: Reduction of at least one grade was noted in 66.0% of IBI311 patients versus 53.3% of placebo, but this difference was not statistically significant (P = .46).
Safety analysis revealed that all adverse events related to infusion reactions, hearing impairment, hyperglycemia, muscle spasms, nausea, or diarrhea were mild or moderate in severity. No serious adverse events or deaths occurred in the IBI311 arm.
Expert Commentary
The RESTORE-1 trial robustly confirms that targeting IGF-1R with IBI311 offers clinically meaningful improvements for Chinese patients with active TED, a group previously understudied. The magnitude of proptosis reduction (-2.85 mm) and CAS improvement is comparable to pivotal teprotumumab trials, reinforcing the mechanistic role of the IGF-1R pathway in TED.
Importantly, this study addresses the racial and geographic gap in TED clinical research. Given phenotypic variations and possible pharmacogenomic differences that can affect efficacy and safety, the positive outcomes in this Chinese cohort support IBI311 as a culturally and regionally appropriate therapeutic alternative.
However, some limitations warrant note. The study excluded patients with sight-threatening disease and those with longstanding TED (>270 days), so results may not generalize to all TED populations. Longer-term data on durability of response and real-world effectiveness are also awaited. Additionally, diplopia outcomes were less robust, suggesting that treatment efficacy for motility disturbances might be more variable.
Conclusion
IBI311 has demonstrated substantial efficacy and a reassuring safety profile in treating active moderate to severe TED among Chinese patients. Its equivalency to teprotumumab in amino acid sequence but distinct dosage form enables expanded accessibility while maintaining therapeutic benefits. Clinicians should consider IBI311 as a promising option addressing an unmet need in Asian TED populations.
Future studies will be valuable to assess long-term outcomes, real-world application, and use in diverse subgroups. Overall, IBI311 represents a significant advancement in TED management, improving patient outcomes and expanding global therapeutic options.
Funding and Trial Registration
This trial was registered at ClinicalTrials.gov with identifier NCT05795621. Funding sources were not explicitly reported in the primary publication.
References
Zhang H, Sun J, Li Y, Zhu L, Shan Z, Lu W, et al. IGF-1R Inhibitor IBI311 for the Treatment of Active Thyroid Eye Disease in Chinese Patients: The RESTORE-1 Randomized Clinical Trial. JAMA Ophthalmol. 2025 Oct 9:e253350. doi:10.1001/jamaophthalmol.2025.3350 IF: 9.2 Q1 . Epub ahead of print. PMID: 41066129 IF: 9.2 Q1 ; PMCID: PMC12512031 IF: 9.2 Q1 .
Additional references on TED and IGF-1R pathway are available in standard endocrinology and ophthalmology literature.