Highlight
– Persistently elevated normalized resting energy expenditure (nREE) measured by indirect calorimetry was independently associated with greater loss of skeletal muscle cross-sectional area (L3 CSA) in critically ill adults.
– Patients classified as hypermetabolic had faster muscle wasting and higher likelihood of energy deficits, and this metabolic phenotype correlated with inflammatory markers rather than sedation or level of consciousness.
– Early, serial indirect calorimetry could identify patients at highest risk and inform targeted nutritional strategies, but interventional data are needed to determine if tailored feeding preserves muscle and improves outcomes.
Background
Loss of skeletal muscle mass during critical illness is common and portends poor outcomes including prolonged mechanical ventilation, disability, and mortality. The mechanisms driving muscle catabolism in the intensive care unit (ICU) are multifactorial—immobility, systemic inflammation, hormonal changes, inadequate nutrition, and altered cellular bioenergetics all contribute. While the clinical importance of preserving lean mass is well recognized, the role of measured whole-body energy expenditure in predicting or mediating muscle wasting has been incompletely characterized.
Indirect calorimetry (IC) provides a bedside measurement of resting energy expenditure (REE) by analyzing oxygen consumption and carbon dioxide production. International expert guidelines (e.g., ESPEN; ASPEN/SCCM statements) recommend IC where available to guide energy prescriptions because predictive equations often mis-estimate actual needs. Yet routinely using IC in the ICU remains variable and the direct relationship between measured metabolic state and objective, radiologically quantified muscle loss has required further study.
Study design
The observational study by von Renesse et al. (Crit Care 2025) included adult ICU patients who underwent at least two indirect calorimetry measurements and had matching abdominal CT scans enabling quantification of posterior muscle cross-sectional area (CSA) at the L3 vertebral level. A subset of patients had three or more paired assessments. Resting energy expenditure was normalized per kilogram body weight (nREE) and patients were categorized by metabolic phenotype (hypermetabolic versus lower metabolic activity). The investigators used regression modeling and group comparisons to assess associations between metabolic state, inflammatory markers, and the degree of muscle loss over time.
Key findings
The cohort comprised 88 patients with at least two IC measurements and corresponding CT scans; 43 patients had three or more assessments. Principal findings were:
- Persistent elevation of nREE was independently associated with greater reductions in L3 posterior muscle CSA. This association held after adjustment for available confounders in multivariable models reported by the authors.
- Patients classified as hypermetabolic by nREE experienced significantly more muscle wasting than those in lower metabolic categories. The manuscript reports the difference in muscle loss as clinically meaningful, though the exact absolute effect sizes should be read in the context of sample size and timing of serial imaging.
- Hypermetabolism correlated with increased inflammatory markers, supporting an inflammatory catabolic phenotype. In contrast, sedation/agitation scales (RAAS) and Glasgow Coma Scale (GCS) were not related to metabolic classification, suggesting consciousness and sedative state did not explain the metabolic differences in this cohort.
- Hypermetabolic patients were at increased risk of cumulative energy deficit, a finding with direct nutritional implications: if energy delivery is not adjusted to meet higher measured needs, these patients are more likely to experience relative underfeeding.
Overall, these data link a measurable metabolic phenotype (raised nREE) with an objective imaging marker of muscle loss, strengthening the biologic plausibility that energy expenditure—and its mismatch with intake—contributes to ICU-acquired lean mass loss.
Clinical interpretation and mechanistic considerations
The observed association between hypermetabolism and muscle wasting aligns with established pathophysiology: systemic inflammation and stress responses increase resting energy expenditure and activate proteolytic pathways in skeletal muscle. Elevated inflammatory cytokines, glucocorticoid signaling, and altered mitochondrial function promote amino acid mobilization from muscle to support gluconeogenesis and acute phase protein synthesis. The study’s finding that inflammatory markers tracked with hypermetabolism supports this paradigm.
From a pragmatic standpoint, measuring REE identifies patients whose actual energy needs exceed predictive equation estimates. If caloric delivery remains standardized or constrained (e.g., due to permissive underfeeding strategies, intolerance, or logistical issues), hypermetabolic patients will accumulate larger energy deficits. Energy shortfalls may exacerbate catabolism and lean mass loss, particularly when protein provision is inadequate to meet increased amino acid requirements.
Why normalization matters—and its limits
The authors normalized REE to body weight (nREE), a common approach that facilitates comparisons across body sizes. However, weight-based normalization can be distorted by fluid accumulation, obesity, and variable proportions of lean mass. In edematous or obese patients, energy needs based on actual body weight may misclassify metabolism; assessing REE in absolute terms and considering lean mass-adjusted metrics can be informative where feasible.
Study strengths
- Integration of serial physiologic (IC) measurements with objective radiologic muscle quantification (L3 CSA), bridging metabolic assessment and structural outcomes.
- Longitudinal design with multiple timepoints in a substantial subgroup, enabling evaluation of persistence of hypermetabolism rather than single-point measures.
- Multivariable analyses to explore independence of the metabolic association with muscle loss.
Limitations and caveats
- Observational design precludes causal inference: hypermetabolism may be a marker of greater illness severity and inflammatory burden that independently drive muscle loss rather than the primary causal factor.
- Selection bias is likely, as inclusion required both multiple IC measurements and CT imaging; such patients may differ from broader ICU populations in ways that affect generalizability.
- Sample size, while reasonable for an imaging-linked ICU cohort, limits precision of effect estimates and subgroup analyses.
- The study report does not fully describe temporal matching of intake data, exact thresholds used to define ‘‘hypermetabolism,’’ or detailed nutrition delivery (caloric and protein intake), which are critical to translate findings into feeding strategies.
- Confounding by fluid status and body composition may affect nREE normalization; edema and obesity can bias per-kilogram metrics.
Implications for practice
This study reinforces guideline recommendations to use indirect calorimetry where available to individualize energy prescriptions in critically ill adults. Serial IC may identify a hypermetabolic phenotype at high risk of lean mass loss and cumulative energy deficit. Clinicians should consider the following practical points:
- When IC is available, measure REE early and repeat when the clinical trajectory changes (e.g., evolving inflammatory state, sepsis resolution, changes in ventilation or sedation).
- Interpret REE alongside assessments of protein needs; preventing muscle catabolism likely requires attention to both energy adequacy and sufficient protein delivery (often 1.2–2.0 g/kg/day depending on guidelines and patient factors).
- Recognize that simply increasing calories without attention to protein, tolerance, glycemia, and the risk of overfeeding may not be beneficial and can cause harm.
- Use bedside tools—nutritional risk scores, ultrasound muscle assessment, and, where available, CT-derived measures—to target patients most likely to benefit from intensified nutrition or anabolic strategies.
Research gaps and future directions
Key unanswered questions include whether tailored increases in energy and protein, guided by serial IC, can reduce muscle loss and improve patient-centered outcomes (functional status, ventilator-free days, mortality). Randomized controlled trials are needed to test feeding strategies stratified by metabolic phenotype. Additional areas for research:
- Mechanistic phenotyping to link cytokine profiles, mitochondrial dysfunction, and proteolytic signaling with measured REE and muscle catabolism.
- Optimization of metabolic normalization methods (lean-mass adjusted REE) and development of pragmatic thresholds for defining hypermetabolism in heterogeneous ICU populations.
- Integration of targeted non-nutritional interventions (early mobilization, anabolic agents) with personalized nutrition for synergistic preservation of muscle.
Expert commentary and guideline context
Current guidelines (ESPEN 2019; ASPEN/SCCM nutrition guidelines) recommend using indirect calorimetry to guide energy provision where resources permit because predictive equations often lack accuracy. This study adds clinically relevant evidence linking measured REE to objective muscle loss and suggests that metabolic phenotyping can identify patients at particularly high risk for catabolism and energy deficit. However, experts caution that measurement alone is not a panacea; appropriate therapeutic trials are required to show that feeding modifications based on IC translate into meaningful clinical benefit.
Conclusion
von Renesse et al. provide important observational evidence that persistent hypermetabolism, measured by serial indirect calorimetry, is independently associated with accelerated CT-quantified muscle loss in critically ill adults and an increased risk of energy deficit. These findings support greater uptake of physiologic metabolic monitoring in the ICU to identify patients who may benefit from individualized nutrition and anti-catabolic strategies. Translation into practice should proceed cautiously and ideally within trials or protocols that monitor not only caloric delivery but also protein adequacy, metabolic tolerance, and patient-oriented outcomes.
Funding and clinicaltrials.gov
The primary publication provided in this summary (von Renesse et al., Crit Care 2025) is cited below. As an observational cohort study, explicit trial registration was not indicated; readers should consult the original article for details on funding and potential conflicts of interest reported by the authors.
References
1. von Renesse J, von Kessel MKF, Oehme F, Kirchberg J, Kalandarishvili M, Nebelung H, Merboth F, Mirtschink P, Weitz J, Distler M, Held HC, Kühn JP, Meisterfeld R. Indirect calorimetry identifies hypermetabolism associated with muscle wasting and increased risk of energy deficit in ICU patients. Crit Care. 2025 Oct 31;29(1):464. doi: 10.1186/s13054-025-05695-y.
2. Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600.
3. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006.
4. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79.
5. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390–438.
Thumbnail image prompt
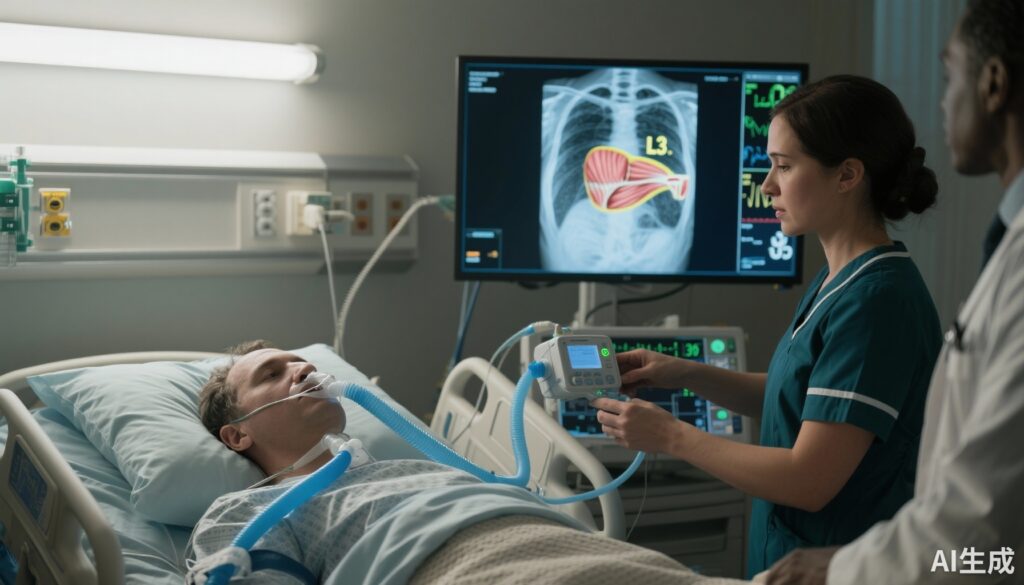
High-resolution, realistic medical illustration: ICU bedside scene showing an intubated adult patient with an indirect calorimetry canopy/device connected, a nurse adjusting the machine, and a computer monitor displaying a color-enhanced CT axial slice of the abdomen highlighting the L3 posterior muscle cross-section; cool-toned color palette, clinical lighting, clear focus on devices and CT image, composed for editorial use.