Highlights
- Development of the UTOpiA system, an integrated extracorporeal circuit combining HLA-depleted human iPSC-derived liver organoids with granulocyte-monocyte apheresis (GMA).
- Demonstration of significant survival benefits and liver function improvements in rat models of acute-on-chronic liver failure (ACLF) and acute liver failure (ALF) using UTOpiA compared to monotherapies.
- Mechanistic insights reveal that iHLC-secreted alpha-fetoprotein (AFP) promotes hepatocyte regeneration by modulating cell cycle arrest and inflammatory cytokines.
- Potential clinical impact as an off-the-shelf liver support system addressing the critical unmet need of hepatic synthetic dysfunction and systemic inflammation in severe liver failure.
Background and Disease Burden
Acute-on-chronic liver failure (ACLF) represents a devastating syndrome characterized by rapid hepatic decompensation in patients with chronic liver disease. This condition carries a dramatically elevated 28-day mortality rate approaching 80%, predominantly due to profound hepatic synthetic failure and systemic inflammatory response syndrome (SIRS). Similarly, acute liver failure (ALF), though distinct in etiology, similarly involves rapid loss of hepatic function and intense systemic inflammation. Current intensive care advances provide supportive but not curative interventions. The absence of effective therapies that simultaneously mitigate systemic inflammation and restore liver synthetic function remains a critical gap in hepatology and critical care medicine, underscoring the urgent need for innovative liver support systems that provide both immunomodulation and functional liver cell replacement.
Study Design and Methodology
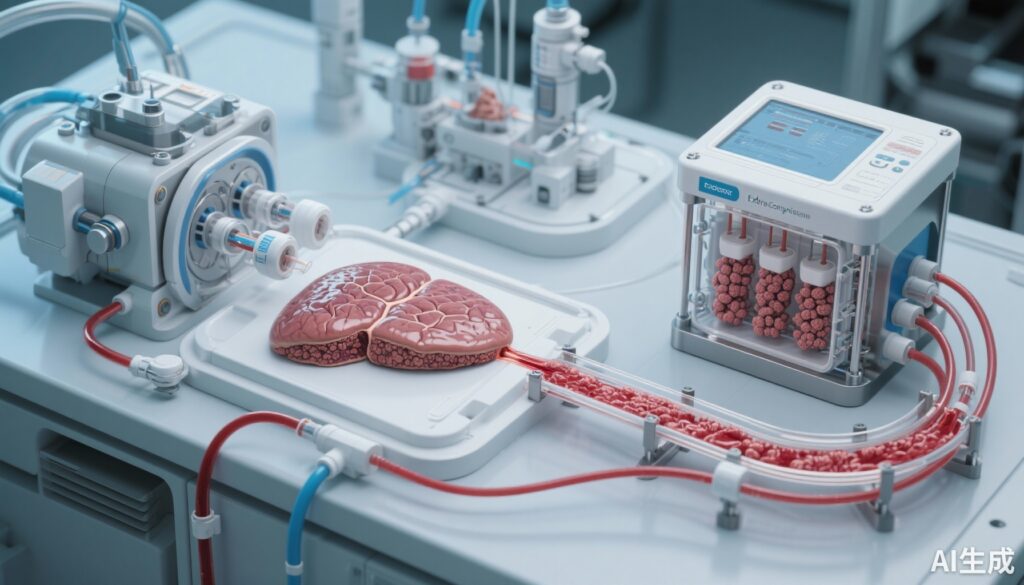
The study by Yamaguchi et al. introduces the UTOpiA system, an extracorporeal therapy combining two novel components: granulocyte-monocyte apheresis (GMA) and HLA-A, B, CIITA triple knockout human induced pluripotent stem cell (iPSC)-derived hepatocyte-like cell (iHLC) organoids. The rationale for this integrated approach is to harness the immunomodulatory capacity of GMA to reduce circulating inflammatory cells and mediators while simultaneously providing synthetic and metabolic liver function via bioengineered, allogeneic-compatible liver organoids.
The experiment was conducted in rat models mimicking ACLF and ALF. Venous whole blood was directly exposed to the UTOpiA extracorporeal circuit allowing interaction with GMA filters and iHLC organoid chambers. Comparator arms included GMA alone, iHLC organoid monotherapy, and conventional HepG2 cell-based devices, enabling assessment of additive or synergistic effects on survival, liver function parameters, inflammatory markers, and liver regeneration.
Key Findings and Results
UTOpiA treatment yielded significant survival advantages in both ACLF and ALF rat models, outperforming all comparator groups including individual GMA or iHLC organoid therapies and HepG2-based devices. Clinical parameters demonstrated marked improvement: diminished encephalopathy reflected by reduced coma scores, normalization of liver biochemistry, and decreased circulating ammonia and bilirubin levels.
Systemic inflammation was substantially suppressed, as evidenced by lowered serum pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), key mediators contributing to the pathophysiology of liver failure and SIRS. Transcriptomic analyses of liver tissue revealed restoration of hepatic metabolic gene expression profiles and increased markers of hepatocyte proliferation and regeneration.
Histopathology corroborated these findings with evidence of hepatocyte regeneration post-treatment. Mechanistic investigations identified that iHLC-secreted alpha-fetoprotein (AFP) plays a pivotal role by downregulating the cyclin-dependent kinase inhibitor p21, thereby alleviating hepatocyte cell cycle arrest and promoting liver regeneration. Additionally, the UTOpiA system appeared to restore the activity of hepatocyte nuclear factor 4 alpha (HNF4α), a critical transcription factor regulating hepatocyte differentiation and metabolic function, further supporting recovery of liver synthetic capabilities.
Expert Commentary
The integrated UTOpiA system represents a significant advancement in liver support technology by concurrently addressing two core pathological drivers of liver failure: inflammation and loss of hepatic synthetic/metabolic function. Previous bioartificial liver systems using hepatoma cell lines or primary hepatocytes have shown limited efficacy and clinical translation barriers due to immunogenicity and lack of functional durability. The triple HLA knockout modification of iPSC derived organoids suggests reduced immunogenicity, offering a potential universal off-the-shelf therapeutic product.
Granulocyte-monocyte apheresis, previously employed in inflammatory bowel disease and other inflammatory conditions, is repurposed here innovatively to modulate systemic inflammation in liver failure. The study’s strong mechanistic data linking AFP secretion to regeneration pathways enriches biological plausibility for the combined approach.
Despite promising preclinical results, translational challenges persist. The rat models, while relevant, do not fully recapitulate human ACLF immunobiology or comorbid conditions. Long-term safety, immunogenicity, and large-animal studies will be critical before clinical application. However, this approach aligns with the current paradigm shift towards precision regenerative medicine in hepatology.
Conclusion and Future Directions
The UTOpiA extracorporeal circuit demonstrates robust preclinical efficacy in reversing ACLF and ALF by combining immunomodulatory apheresis with HLA-depleted iPSC-derived liver organoids that provide metabolic and synthetic liver functions as well as pro-regenerative signals. This dual mechanism addresses urgent unmet clinical needs in severe liver failure management where limited therapeutic options currently exist.
If successfully translated to humans, the UTOpiA system could become an off-the-shelf, scalable liver support therapy improving survival and recovery while potentially bridging patients to liver transplantation or allowing liver regeneration. Future clinical trials should focus on safety, dosing parameters, and efficacy in human ACLF and ALF populations, along with mechanistic exploration of hepatic regeneration pathways in diverse etiologies.
In summary, this innovative approach marks an important step towards integrative extracorporeal liver support by targeting both systemic inflammation and hepatic synthetic/metabolic recovery, offering hope for improved outcomes in critical liver failure syndromes.
Funding and Clinicaltrials.gov
No specific funding information was provided in the original publication. Clinical trial registration for translational studies has not been reported as of this publication.
References
Yamaguchi H, Yoneyama Y, Ichimura K, et al. Reversal of ACLF and ALF using whole blood extracorporeal system combining HLA-depleted liver organoids with granulocyte-monocyte apheresis. J Hepatol. 2025 Oct 2:S0168-8278(25)02477-8. doi:10.1016/j.jhep.2025.08.038. Epub ahead of print. PMID: 41044028.