Introduction
Metastatic castration-resistant prostate cancer (mCRPC) remains a formidable clinical challenge and a leading cause of cancer mortality among men globally. Despite advances in systemic therapies such as androgen receptor pathway inhibitors (ARPIs) including enzalutamide and abiraterone, survival outcomes remain suboptimal, particularly when bone metastases are present. Radium-223 dichloride (Ra223), an alpha particle-emitting radiopharmaceutical that selectively targets osteoblastic bone metastases, has previously demonstrated survival benefit when used as monotherapy in symptomatic mCRPC patients. However, combination strategies with ARPIs have encountered safety concerns as noted in the ERA 223 trial where Ra223 was combined with abiraterone, leading to increased fracture rates and mortality. The EORTC 1333/PEACE-3 trial investigates the combination of enzalutamide with Ra223 as first-line therapy in mCRPC with bone metastases, with enforced use of bone-protecting agents (BPAs) to mitigate skeletal adverse events.
Study Design
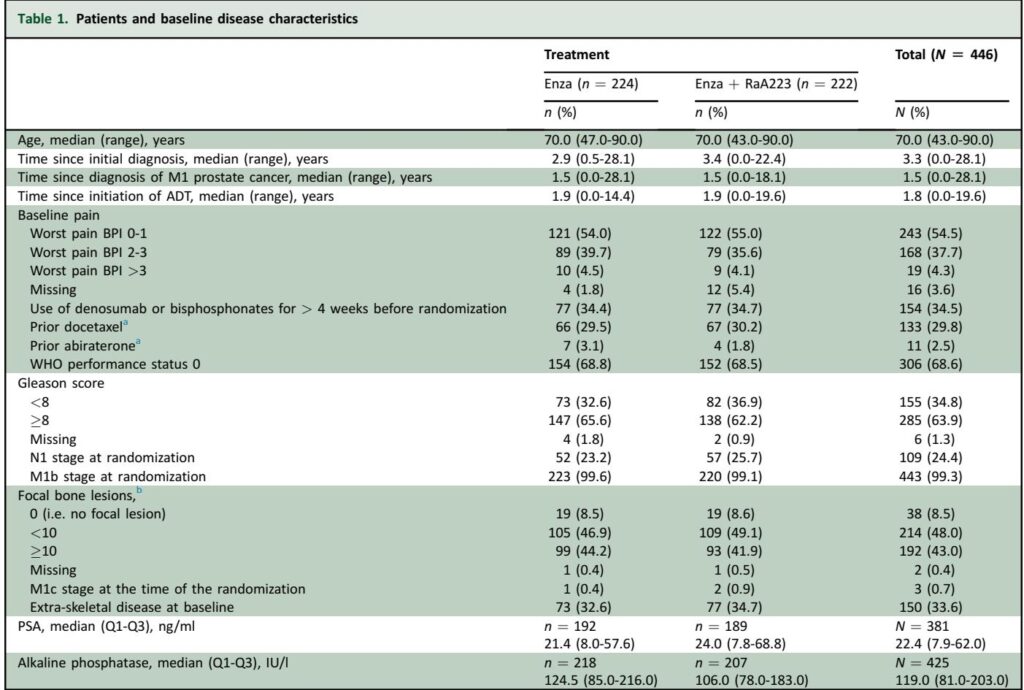
PEACE-3 is a multinational, randomized, open-label phase III trial enrolling 446 patients with progressive mCRPC and at least two bone metastases (increased to four in Europe following protocol amendment). Patients without visceral metastases and with asymptomatic or mild pain were randomized 1:1 to receive either enzalutamide 160 mg daily monotherapy or enzalutamide combined with six monthly intravenous injections of Ra223 at 55 kBq/kg. Mandatory concomitant BPA use (zoledronic acid or denosumab) was instituted starting March 2018 based on prior safety signals. Stratification factors included country, baseline pain score, prior docetaxel exposure, BPA usage, and prior abiraterone. The primary endpoint was radiological progression-free survival (rPFS) assessed by investigator. Key secondary endpoints comprised overall survival (OS), time to next systemic treatment (TTNT), time to pain progression (TTPP), and time to symptomatic skeletal event (TTSSE). Safety assessments encompassed clinical, laboratory, and adverse event monitoring according to CTCAE v4.03 standards.
Key Findings
With median follow-up exceeding 41 months, the trial met its primary endpoint. The addition of Ra223 to enzalutamide significantly improved rPFS: median rPFS was 19.4 months (95% CI 17.1–25.3) in the combination arm versus 16.4 months (95% CI 13.8–19.2) with enzalutamide alone (HR 0.69; 95% CI 0.54–0.87; P=0.0009). This treatment effect was consistent across all prespecified subgroups without significant interaction.
At a preplanned interim OS analysis at 80% of events, the combination arm demonstrated a median OS of 42.3 months (95% CI 36.8–49.1) compared to 35.0 months (95% CI 28.8–38.9) in the monotherapy arm (HR 0.69; 95% CI 0.52–0.90; P=0.0031). Although the proportional hazards assumption was violated due to early deaths in the combination arm, longer follow-up is planned to confirm this promising survival benefit.
TTNT was significantly prolonged with the combination (HR 0.57; P<0.0001), whereas no significant difference was observed for TTPP or TTSSE at the interim analysis.
Safety profiles were consistent with expectations: grade ≥3 treatment-emergent adverse events (TEAEs) occurred in 55.8% of patients on enzalutamide alone and 65.6% receiving combined treatment. Notably, the combination increased the incidence of fractures (24.3% vs. 13.4%) despite mandatory BPA use, with fracture rates decreasing substantially after implementation of this requirement. Hypertension emerged as a common grade ≥3 TEAE (~34% in both arms), higher than previously reported with enzalutamide, possibly due to broader patient inclusion criteria.
Expert Commentary
PEACE-3 represents a rigorous academic effort to address a critical unmet need in mCRPC management: improving outcomes for patients with bone metastases while maintaining safety. The significant improvement in rPFS and the promising OS trend underscore the potential additive or synergistic benefits of Ra223 with enzalutamide, possibly reflecting Ra223’s disruption of the bone metastatic niche through complex mechanisms beyond direct cytotoxicity.
The non-proportional hazard observed in OS analysis, with early cluster deaths, warrants cautious interpretation but may reflect immunomodulatory or delayed effects analogous to those observed in immunotherapy trials where OS benefits exceed rPFS improvements. This finding justifies the planned final OS analysis at full event accrual to robustly define long-term benefit.
Mandatory BPA use was vital to reduce skeletal complications. PEACE-3 confirms prior safety signals from ERA 223 and highlights the necessity of bone health management in this context. Continued vigilance for fractures and osteonecrosis of the jaw remains imperative.
Limitations include the lengthy enrollment period (>8.5 years), reflecting shifting clinical practices and regulatory changes following ERA 223. The small minority of patients pretreated with ARPIs in hormone-sensitive settings may limit generalizability to contemporary populations increasingly receiving earlier ARPI therapy. Nevertheless, enzalutamide plus Ra223 remains relevant, particularly for patients with limited prior ARPI exposure or contraindications.
Conclusion
The EORTC 1333/PEACE-3 trial establishes that adding six cycles of Ra223 to enzalutamide and BPAs significantly improves rPFS and suggests an OS advantage in patients with mCRPC and bone metastases. The combination’s safety profile is manageable, with emphasis on fracture prevention through bone-targeted agents. This regimen offers a compelling first-line treatment option, highlighting the importance of integrating radiopharmaceuticals with ARPIs in mCRPC therapeutic sequencing. Ongoing follow-up will clarify the magnitude and durability of survival benefit.
References
Tombal B, Choudhury A, Saad F, et al. Enzalutamide plus radium-223 in metastatic castration-resistant prostate cancer: results of the EORTC 1333/PEACE-3 trial. Ann Oncol. 2025 Sep;36(9):1058-1067. doi:10.1016/j.annonc.2025.05.011 IF: 65.4 Q1 . Epub 2025 May 30. PMID: 40450503 IF: 65.4 Q1 .