Highlight
- Anogenital basal cell carcinomas (BCCs) predominantly affect sun-protected areas such as the vulva and perianal region, differing from typical UV-exposed BCCs.
- These tumors exhibit low mutational burden, absence of ultraviolet (UV) mutational signatures, and frequent PTCH1 mutations, confirming sonic hedgehog (SHH) pathway dependence.
- A notable 15% local recurrence rate over seven years indicates higher recurrence than UV-induced BCCs, underlining the need for vigilant clinical follow-up.
- HPV infection appears uninvolved despite some p16 expression, with some vulvar BCCs arising on lichen sclerosus, suggesting distinct pathogenesis.
Study Background and Disease Burden
Basal cell carcinoma is the most common form of skin cancer, classically arising on sun-exposed skin due to cumulative ultraviolet radiation damage. However, anogenital BCCs are a rare subtype occurring in sun-protected areas, such as the vulva, perianal and genital regions. Their rarity has limited understanding of their clinical behavior, genomic landscape, and risk factors. The burden of disease in these sites includes challenges in early diagnosis, treatment complexity due to anatomical and functional considerations, and higher concern about local recurrence impacting quality of life. Furthermore, entities like lichen sclerosus, a chronic inflammatory dermatosis that affects anogenital skin and is associated with squamous cell carcinoma risk, may contribute to tumorigenesis in this context. Understanding the clinical and molecular characteristics of anogenital BCCs is critical for tailoring management and surveillance strategies distinct from typical UV-associated BCCs.
Study Design
This retrospective-prospective cohort study was performed in France, encompassing 50 patients diagnosed with anogenital BCCs from 2006 to 2024. The cohort was predominantly elderly (mean age 78 years) and female (80%). Researchers extensively reviewed clinical presentations including tumor location, histological subtype, size (mean 4.1 cm), and prior history of radiation exposure.
Treatment modalities were primarily surgical excision alone (84% of patients), with a minority receiving neoadjuvant hedgehog pathway inhibitors (6%) to reduce tumor burden prior to surgery. Outcomes included local recurrence, strictly defined as new tumors developing within the prior BCC surgical scar. Additionally, HPV testing and histological examination for lichen sclerosus were conducted to assess potential pathogenic contributors.
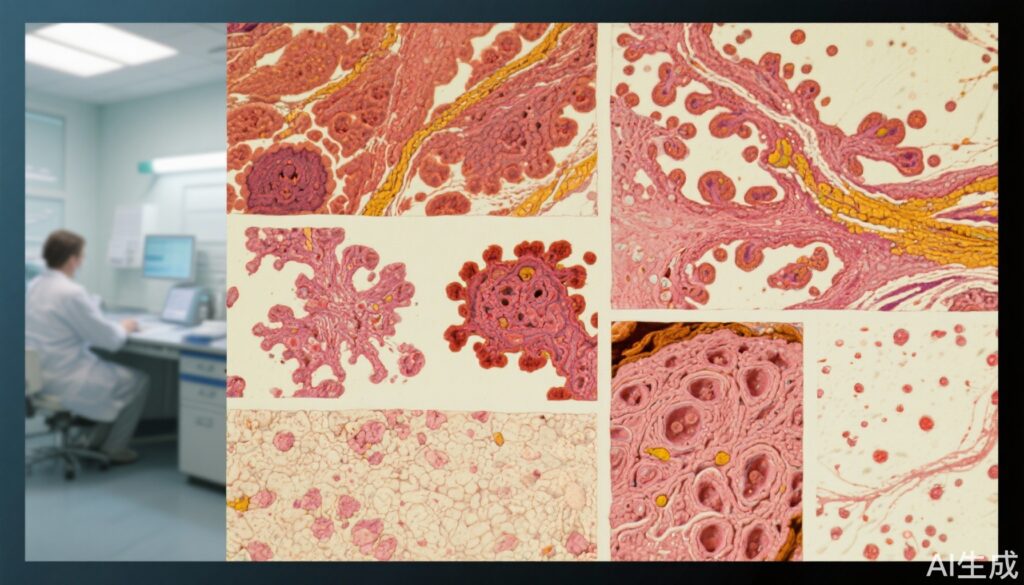
A subset of tumors (n=12) and matched non-tumoral tissues underwent genomic profiling to interrogate mutational burden, presence of driver mutations, UV signature mutations, and pathway dependencies.
Key Findings
The anogenital BCCs predominantly involved the vulva in women (34 of 50 cases) and the perianal region in men (5 cases). Approximately 18% of vulvar BCCs developed on background histologically confirmed lichen sclerosus, implicating a possible role of chronic dermatosis in tumorigenesis.
HPV testing was negative in all cases, including those with aberrant p16 immunostaining, suggesting that HPV is not an etiologic factor for these tumors—a contrast to anogenital squamous cell carcinomas.
Among the cohort, three patients with prior radiation therapy had tumors arising within previously irradiated fields, indicating radiation as a potential risk factor in select cases.
Histologically, tumors were nearly evenly split between nodular (46%) and infiltrative (45%) subtypes. The mean tumor size was notably large at 4.1 cm, reflecting possible delayed diagnosis or more aggressive behavior.
Regarding clinical outcomes, local recurrence occurred in 15% (7 of 48 treated patients) over a median follow-up of 7 years. This recurrence rate is higher than typically observed in sun-exposed BCCs, consistent with a distinct clinical course.
Genomic analysis revealed a low tumor mutational burden across the sampled tumors, which lacked UV-related mutational signatures that typify classic BCCs. TP53 mutations, frequent in UV-induced BCCs, were rare (two cases), while PTCH1 mutations were frequent (nine of 12 cases), reinforcing the pivotal role of aberrant sonic hedgehog (SHH) pathway activation in anogenital BCC pathogenesis.
These genomic findings confirm that anogenital BCCs, while genetically dependent on SHH pathway dysregulation, differ fundamentally from UV-driven BCCs in their mutational landscape.
Expert Commentary
This comprehensive clinical and molecular characterization of anogenital BCCs clarifies key distinctions from their sun-exposed counterparts. The absence of UV mutational signatures and limited TP53 alterations suggest a fundamentally different carcinogenesis mechanism, likely driven by SHH pathway activation resulting from PTCH1 mutations.
Association with lichen sclerosus in a subset of cases opens intriguing questions regarding chronic inflammation as a promoting factor for tumor development, a hypothesis supported by the known pro-carcinogenic microenvironment in lichen sclerosus. Negative HPV results discount viral oncogenesis, contrasting with other anogenital malignancies.
The higher local recurrence rate emphasizes the need for rigorous post-treatment surveillance and possibly innovative multimodal therapies. The use of hedgehog inhibitors as neoadjuvant therapy in a minority of cases offers a promising approach but warrants further evaluation in prospective trials.
Limitations of the study include the relatively small genomic sample size and the retrospective design; however, the prospective elements and long follow-up provide valuable insight. Future research should aim to corroborate these findings in larger, multicenter cohorts and explore therapeutic implications of these molecular differences.
Conclusion
Anogenital basal cell carcinomas constitute a rare but clinically significant entity distinct from UV-induced BCCs. They predominantly affect elderly women’s vulvar area, can arise on lichen sclerosus, and exhibit a higher local recurrence risk after treatment.
Molecular profiling underscores a low mutational burden, lack of UV-related mutations, and frequent PTCH1 mutations that drive sonic hedgehog pathway dysregulation. These insights suggest the need for specialized diagnostic, therapeutic, and surveillance strategies customized to their unique biology and clinical behavior.
Clinicians should be vigilant in managing anogenital BCCs, considering their aggressive recurrence profile and divergent pathogenesis, to optimize outcomes and preserve anogenital function.
References
Dousset L, Yurchenko AA, Chanal J, Beylot-Barry M, Samimi M, Chabane D, Aractingi S, Nassar D, Alkobtawi M, Quereux G, Berard F, Geffrier C, Plantier F, Ly S, Herms F, Jullie ML, Jouary T, Lamant L, Battistella M, Nikolaev SI, Basset-Seguin N. Genomic and clinical characterization of anogenital basal cell carcinoma: a retrospective and prospective cohort study. J Am Acad Dermatol. 2025 Aug 23:S0190-9622(25)02680-5. doi: 10.1016/j.jaad.2025.08.054. Epub ahead of print. PMID: 40854498.