Highlights
- Large outbreak of toxigenic Corynebacterium diphtheriae among migrants in Europe, predominantly manifesting as cutaneous disease.
- Genomic analysis revealed multiclonal origins and cross-border transmission, with substantial antimicrobial resistance detected.
- Low ascertainment of vaccination status and significant clinical burden, including coinfections, complicated management.
- Antimicrobial resistance to erythromycin and penicillin was observed, threatening the efficacy of first-line treatments.
Clinical Background and Disease Burden
Diphtheria, caused by toxigenic strains of Corynebacterium diphtheriae, is a potentially severe infectious disease characterized by local and systemic effects, including pseudomembrane formation in the respiratory tract and cutaneous ulcerations. While largely controlled in high-income countries through routine vaccination, the disease remains a public health threat in settings with low vaccine coverage and among vulnerable populations.
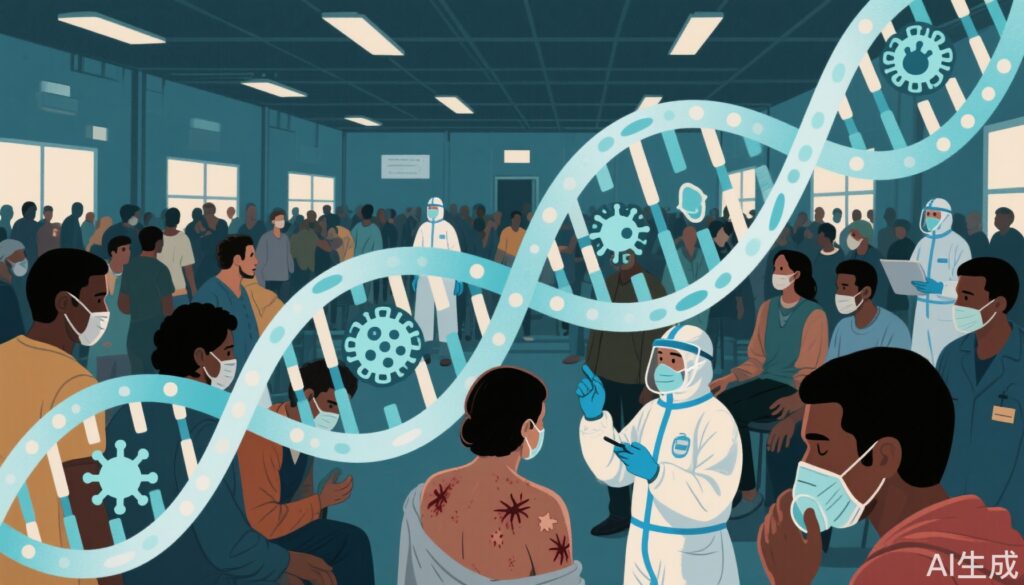
In the summer of 2022, a surge of diphtheria cases was observed in migrant reception centers across Europe. These settings, often marked by overcrowding and limited healthcare access, provide fertile ground for the resurgence of vaccine-preventable diseases. The outbreak prompted the formation of a pan-European consortium to systematically assess the clinical, epidemiologic, and microbiologic features of these cases.
Research Methodology
The consortium conducted a multicountry, observational study across 10 European nations, including Germany, Austria, Switzerland, the United Kingdom, and others. The study period spanned January to November 2022. All reported cases of toxigenic C. diphtheriae were included, and clinical data were extracted where available.
Patient interviews provided information on countries of origin and migration transit routes. Laboratory investigation included whole-genome sequencing of isolates and antimicrobial susceptibility testing. Phylogenetic relationships and resistance gene profiles were characterized to map transmission dynamics and resistance threats.
Key Findings
Among 362 patients, 363 toxigenic C. diphtheriae isolates were identified. The majority of patients (77.5%) presented with cutaneous diphtheria, while 15.3% had respiratory involvement and 2.6% showed both. Pseudomembrane formation, a hallmark of respiratory diphtheria, was observed in 11 cases, and one patient with a pseudomembrane died following multiorgan failure after delayed antitoxin administration.
Vaccination status was largely unascertained: only 4 patients were confirmed vaccinated, 10 unvaccinated, and 290 unknown. This uncertainty underscores the vulnerability of migrant populations to vaccine-preventable infections. Coinfections with Streptococcus pyogenes, methicillin-resistant Staphylococcus aureus (MRSA), and arcanobacteria were common, especially in cutaneous cases. Scabies infestations were also reported.
Antibiotic usage varied, with azithromycin, amoxicillin, and clarithromycin most frequently prescribed. Notably, antimicrobial resistance was significant: isolates harboring the ermX gene were resistant to erythromycin, while those with pbp2m and blaOXA-2 genes showed beta-lactam resistance. Penicillin resistance was present, but amoxicillin retained efficacy against pbp2m carriers.
Whole-genome sequencing revealed four major genetic clusters, indicating multiclonal outbreak origins with repeated cross-border spread. The most recent common ancestors of these clusters dated from 2017 to 2020, suggesting persistent transmission and reintroduction into Europe.
Most patients were managed on site; however, 17 required hospitalization (5 with cutaneous, 12 with respiratory diphtheria). All hospitalized respiratory cases received diphtheria antitoxin and antibiotics, with most treated promptly. Delayed antitoxin administration in one fatal case highlighted the importance of early recognition and intervention.
Mechanistic Insights and Biological Plausibility
Diphtheria toxin is the principal virulence factor, causing local cytotoxicity and systemic complications. The cutaneous form, which predominated in this outbreak, likely reflects transmission via skin-to-skin contact in crowded settings. The detection of multiclonal lineages and antimicrobial resistance genes supports ongoing evolution and adaptation of C. diphtheriae in response to antibiotic pressure and population movement.
Emergence of resistance, particularly to erythromycin and penicillin, is a critical concern given reliance on these agents for first-line therapy. The finding that pbp2m-positive isolates remain susceptible to amoxicillin suggests potential therapeutic alternatives but underscores the need for susceptibility-guided treatment.
Controversies and Limitations
Incomplete clinical documentation and unreliable vaccination records among migrants hampered full assessment of risk factors and vaccine effectiveness. The observational nature of the study precludes inference regarding causality or effectiveness of specific interventions. Coinfections and comorbidities, common in this population, may confound clinical outcomes.
Generalizability to non-migrant populations or other geographic contexts is limited, but the findings highlight broader challenges in infectious disease control amid humanitarian crises and cross-border migration.
Conclusion
This multicountry outbreak of C. diphtheriae among migrants in Europe underscores the persistent threat posed by vaccine-preventable diseases in vulnerable populations. The multiclonal spread, high rates of cutaneous infection, and significant antimicrobial resistance highlight urgent needs for improved surveillance, vaccination strategies, rapid diagnostics, and tailored antimicrobial stewardship. Early recognition and timely administration of diphtheria antitoxin are critical for optimal outcomes. Ongoing genomic surveillance and cross-border coordination will be essential to contain similar outbreaks in the future.
References
Hoefer A, Seth-Smith H, Palma F, Schindler S, Freschi L, Dangel A, Berger A, D’Aeth J, Cordery R, Delgado-Rodriguez E, Gruner E, Flury D, Hinic V, Kofler J, Lienhard R, Mariman R, Nolte O, Schibli A, Toubiana J, Traugott M, Jacquinet S, Indra A, Fry NK, Palm D, Sing A, Brisse S, Egli A; 2022 European Diphtheria Consortium. Corynebacterium diphtheriae Outbreak in Migrant Populations in Europe. N Engl J Med. 2025 Jun 19;392(23):2334-2345. doi: 10.1056/NEJMoa2311981.