Introduction
Peripheral artery disease (PAD) is a common and debilitating vascular complication among individuals with type 2 diabetes, characterized by atherosclerosis that impairs lower extremity perfusion, leading to reduced functional capacity, increased risk of limb events, and diminished quality of life. Despite its high prevalence and impact, therapeutic options targeting functional impairment in symptomatic PAD remain limited, particularly therapies that address metabolic comorbidities such as diabetes and obesity. The Semaglutide and Walking Capacity in People with Symptomatic Peripheral Artery Disease and Type 2 Diabetes (STRIDE) trial was designed to assess the effects of semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1 RA), on walking function and symptom burden in this population.
Study Design and Methods
STRIDE (NCT04560998) was a multinational, randomized, double-blind, placebo-controlled trial enrolling 792 participants with symptomatic PAD (Fontaine classification IIa) and type 2 diabetes, conducted across 112 sites in 20 countries. Key inclusion criteria included adults ≥18 years with an ankle-brachial index ≤0.90 or toe-brachial index ≤0.70, stable on background medications, and without recent cardiovascular or revascularization events.
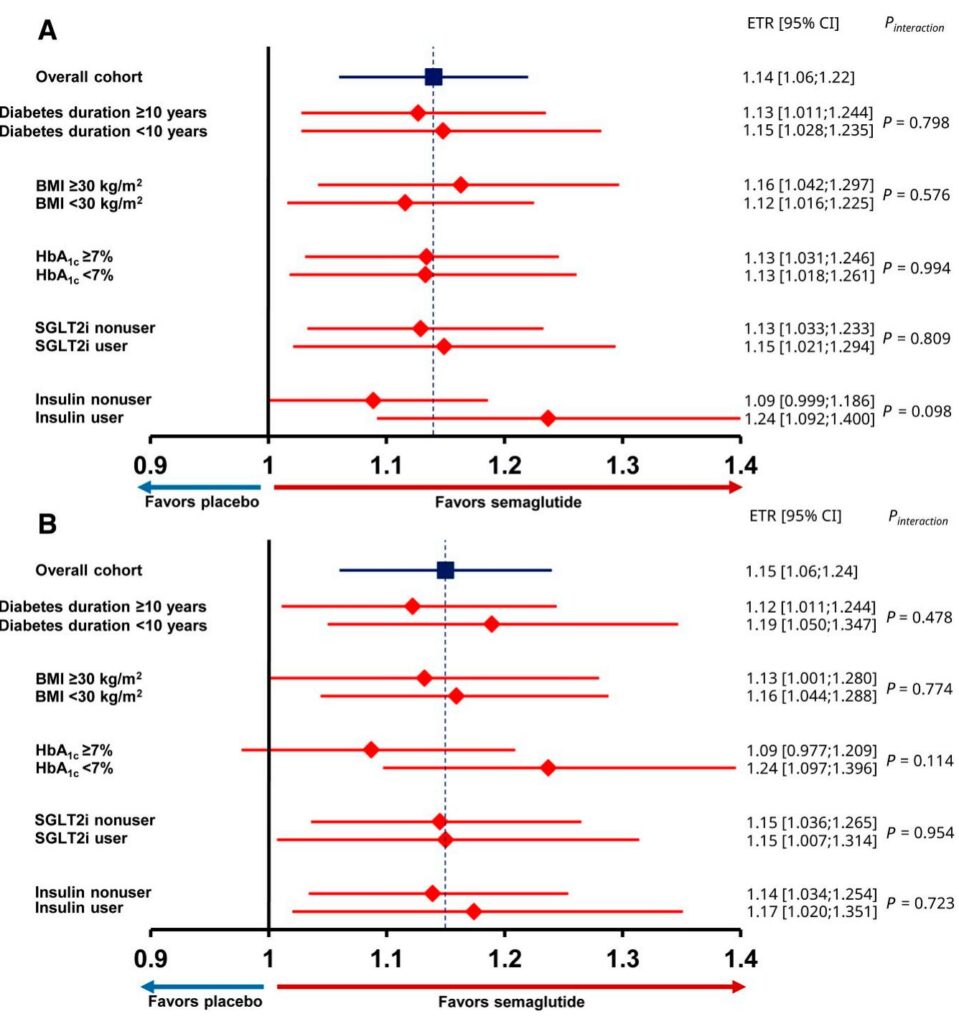
Participants were randomized to receive once-weekly subcutaneous semaglutide 1.0 mg or matched placebo for 52 weeks. The primary endpoint was the ratio to baseline in maximum walking distance (MWD) assessed by a constant load treadmill test at week 52. A key secondary endpoint was the ratio to baseline in pain-free walking distance (PFWD) similarly assessed. Subgroup analyses examined the consistency of treatment effects across baseline diabetes characteristics including diabetes duration (<10 years vs. ≥10 years), body mass index (BMI <30 vs. ≥30 kg/m2), glycemic control defined by HbA1c (<7% vs. ≥7%), and concomitant use of sodium–glucose cotransporter 2 inhibitors (SGLT2i) or insulin.
A mixed model for repeated measurements was employed, integrating treatment, region, and subgroup fixed factors and baseline covariates, with estimation of treatment-by-subgroup interactions. Safety outcomes were monitored throughout the trial.
Key Findings
Baseline demographics showed a median diabetes duration of 12.2 years, mean HbA1c of 7.1%, and mean BMI of 28.7 kg/m2. Approximately 35.1% used SGLT2 inhibitors and 31.7% used insulin.
Semaglutide significantly improved MWD relative to placebo with an estimated treatment ratio (ETR) of 1.14 (95% CI, 1.06–1.22; P=0.0005), corresponding to an average increase in MWD of 22.5 m in participants with BMI <30 kg/m2 and 62.9 m in those with BMI ≥30 kg/m2. Notably, the treatment benefit was consistent regardless of diabetes duration (ETR 1.15 vs. 1.13 for <10 vs. ≥10 years; P=0.80), BMI category (1.12 vs. 1.16; P=0.58), HbA1c status (both groups ETR 1.13; P=0.99), or concomitant SGLT2i or insulin use.
Similarly, PFWD improved significantly with semaglutide across all subgroups (overall ETR 1.15; 95% CI 1.06–1.24; P=0.0007). Effect sizes were consistent with MWD findings, and no statistically significant treatment-by-subgroup interactions were observed, indicating uniform benefit.
Weight reduction was more pronounced with semaglutide (mean difference ~4 kg) compared to placebo and was greater among participants with higher baseline BMI. However, the correlation between weight loss and improvement in walking distance was weak (Spearman r ≈ -0.12, P=0.03), and no significant correlation was found between HbA1c reduction and walking improvements, suggesting that the beneficial effects of semaglutide on walking capacity extend beyond weight loss and glycemic control.
Adverse event rates, including gastrointestinal events and hypoglycemia, were comparable across treatment groups and subgroups, with no new safety concerns identified.
Expert Commentary
This analysis of STRIDE reinforces semaglutide’s robust efficacy in enhancing functional walking capacity among a heterogeneous population with symptomatic PAD and type 2 diabetes. The consistent benefits across subgroups defined by duration of diabetes, BMI, HbA1c, and concurrent diabetes therapies underscore its broad applicability in clinical practice. Of particular interest is the independence of walking improvements from glycemic and weight changes, supporting novel mechanisms such as improved endothelial function, microvascular perfusion, anti-inflammatory effects, and arterial stiffness reduction—all mediated by GLP-1 receptor activation.
Given the burden of PAD on mobility and quality of life, and the scarcity of effective pharmacotherapies, semaglutide’s dual role in addressing metabolic dysfunction and functional impairment is promising. Its proven cardiovascular and renal protective effects further augment its utility in this complex patient population. Clinicians currently recommended to screen for asymptomatic PAD in diabetics aged ≥65 years or with ≥10 years duration may consider earlier intervention with semaglutide to preserve functional status and potentially mitigate disease progression.
Limitations include the exploratory nature of subgroup analyses, possible underpowering for interaction tests, and absence of multiplicity adjustments. Future studies should elucidate mechanistic pathways, long-term limb outcomes, and combination strategies with revascularization and rehabilitation.
Conclusions
Once-weekly subcutaneous semaglutide 1.0 mg significantly improves maximum and pain-free walking distances in patients with symptomatic PAD and type 2 diabetes, irrespective of baseline diabetes duration, BMI, glycemic control, or diabetes medication use. These findings support semaglutide as an effective therapeutic option across diverse metabolic phenotypes, offering functional benefits beyond glycemic and weight effects. Incorporation of semaglutide into comprehensive PAD management may enhance mobility, quality of life, and cardiovascular health in this high-risk population.
Reference
Rasouli N, Guder Arslan E, Catarig AM, Houlind K, Ludvik B, Nordanstig J, Sourij H, Thomas S, Verma S, Bonaca MP. Benefit of Semaglutide in Symptomatic Peripheral Artery Disease by Baseline Type 2 Diabetes Characteristics: Insights From STRIDE, a Randomized, Placebo-Controlled, Double-Blind Trial. Diabetes Care. 2025 Sep 1;48(9):1529-1535. doi: 10.2337/dc25-1082. PMID: 40543068; PMCID: PMC12368379.