Highlight
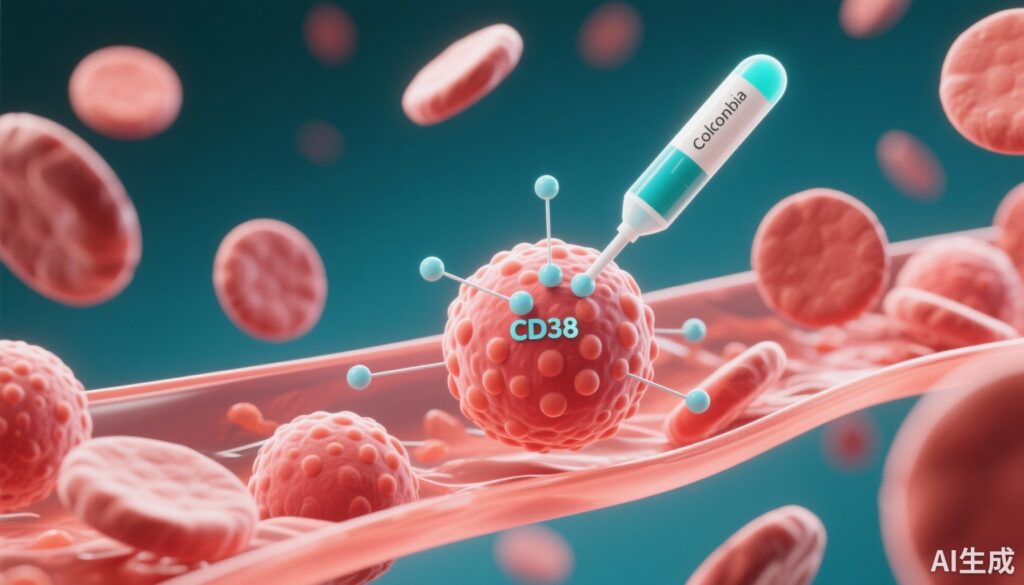
This multicentre, randomized, placebo-controlled phase 2 trial evaluated CM313, an anti-CD38 monoclonal antibody, in adults with persistent or chronic primary immune thrombocytopenia (ITP) refractory or relapsed after glucocorticoid therapy. Compared with placebo, CM313 markedly increased the overall platelet response rate at week 8 (83% vs 20%; P<0.001) and shortened the time to achieving target platelet counts. The treatment demonstrated a favorable safety profile with primarily infusion-related reactions and petechiae as common adverse events.
Study Background: Disease Burden and Unmet Need
Primary immune thrombocytopenia is an autoimmune disorder characterized by immune-mediated destruction and insufficient production of platelets, leading to bleeding risk. Despite first-line treatments such as glucocorticoids, a significant proportion of patients experience persistent or chronic disease with suboptimal response or relapse, challenging clinical management. Current second-line options, including thrombopoietin receptor agonists, immunosuppressants, or splenectomy, have variable efficacy and safety concerns. Novel targeted therapies that can improve platelet count rapidly and sustain durable responses with manageable toxicity remain greatly needed.
Study Design
This phase 2, multicentre, randomized, double-blind, placebo-controlled trial was conducted across five hospitals in China from January to June 2024. Forty-five adult patients (≥18 years) with persistent or chronic primary ITP, who had failed to respond to or relapsed after glucocorticoids but had previously responded to first-line therapy, were enrolled. Participants were randomized 2:1 to receive intravenous CM313 at 16 mg/kg or placebo weekly for eight weeks. The primary endpoint was overall response rate at week 8, defined as having at least two consecutive platelet counts ≥30×109/L, a minimum doubling from baseline, and absence of bleeding. Secondary endpoints included time to first two consecutive platelet counts ≥50×109/L and duration of response. Safety outcomes encompassed treatment-emergent adverse events.
Key Findings
Among 56 screened patients, 30 received CM313 and 15 received placebo. Baseline characteristics were balanced. At week 8, overall response rate was significantly higher in the CM313 arm (83%; 25/30) compared to placebo (20%; 3/15), with an absolute difference of 63.3% (95% CI 33.7% to 81.3%; P<0.001). The median time to achieve platelet counts ≥50×109/L was one week in the CM313 group versus unreached in placebo (P<0.001), indicating faster platelet recovery with CM313. Furthermore, the median cumulative response duration was longer with CM313 (18 weeks) compared to placebo (3 weeks) (P=0.004), suggesting sustained efficacy beyond treatment period.
Regarding safety, treatment-emergent adverse events occurred in 87% of CM313-treated patients and 80% in placebo. Most events were mild or moderate. The most common adverse effects with CM313 included infusion-related reactions and petechiae, consistent with expected immune and hematological effects. No new safety signals or treatment-related severe adverse events were reported, underscoring a manageable safety profile.
Expert Commentary
The reported results provide compelling evidence that targeting CD38 with CM313 is a promising therapeutic approach in immune thrombocytopenia, a disease historically difficult to treat in refractory or chronic stages. CD38 is expressed on immune cells implicated in autoantibody production and destruction of platelets. By depleting or modulating such pathogenic immune cells, CM313 facilitates rapid platelet recovery while maintaining safety. Its rapid onset and sustained responses compare favorably with existing second-line options, potentially filling a critical therapeutic gap.
However, limitations include the relatively small sample size and limited follow-up to fully assess long-term durability and rare adverse events. Additionally, the study population was exclusively Chinese, which may affect generalizability to other ethnic groups. Larger phase 3 trials are warranted to confirm efficacy, evaluate diverse patient populations, and compare CM313 head-to-head with established treatments.
Current guideline recommendations for ITP emphasize individualized treatment balancing bleeding risk and adverse effects. If larger confirmatory studies validate these findings, CM313 may emerge as a valuable addition, particularly for patients with persistent or chronic disease failing first-line therapy.
Conclusion
In this phase 2 trial, CM313 showed marked efficacy in improving platelet counts rapidly and sustaining response in adults with persistent or chronic primary immune thrombocytopenia refractory or relapsed after glucocorticoid treatment. The treatment was well tolerated with manageable adverse events. These findings support further development of CM313 as a novel immunotherapy offering a promising therapeutic option in ITP management.
Funding and Clinical Trial Registration
The trial was registered at ClinicalTrials.gov (NCT06199089). Funding sources were not explicitly stated in the report.
References
Chen Y, Xu Y, Dai J, et al. Anti-CD38 monoclonal antibody CM313 for primary immune thrombocytopenia: multicentre, randomised, placebo controlled, phase 2 trial. BMJ. 2025 Oct 21;391:e084314. doi: 10.1136/bmj-2025-084314. PMID: 41120215; PMCID: PMC12538382.