Introduction
Myocarditis and pericarditis are inflammatory diseases affecting the myocardium and pericardium, respectively. While often recognized in younger individuals, epidemiological data reveal incidence across all age groups. These conditions have diverse etiologies, including viral infections, immune-mediated inflammatory diseases (IMIDs), and malignancy-related factors; however, a substantial fraction of cases is idiopathic. Clonal hematopoiesis of indeterminate potential (CHIP) reflects age-associated expansion of hematopoietic stem cells carrying leukemia-linked mutations without overt malignancy. Commonly involving DNMT3A and TET2 genes, CHIP contributes to cardiovascular disease through inflammatory pathways, yet its association with inflammatory cardiac conditions remains understudied.
Study Design and Methods
This observational cohort study analyzed data from the UK Biobank, enrolling 335,426 participants aged approximately 56 years on average, free of baseline cardiovascular or hematological malignancy, and with whole-exome sequencing data. CHIP exposures included any clone with variant allele frequency (VAF) ≥2% and large clones with VAF ≥10%, with secondary focus on DNMT3A and TET2 mutations. The primary outcome was incident myocarditis or pericarditis identified by diagnostic codes over a median follow-up of 13.6 years. Multivariable Cox regression models adjusted for demographics and cardiovascular risk factors tested associations. Secondary analyses examined myocarditis and pericarditis separately and compared findings to other cardiovascular diseases and IMIDs.
Key Findings
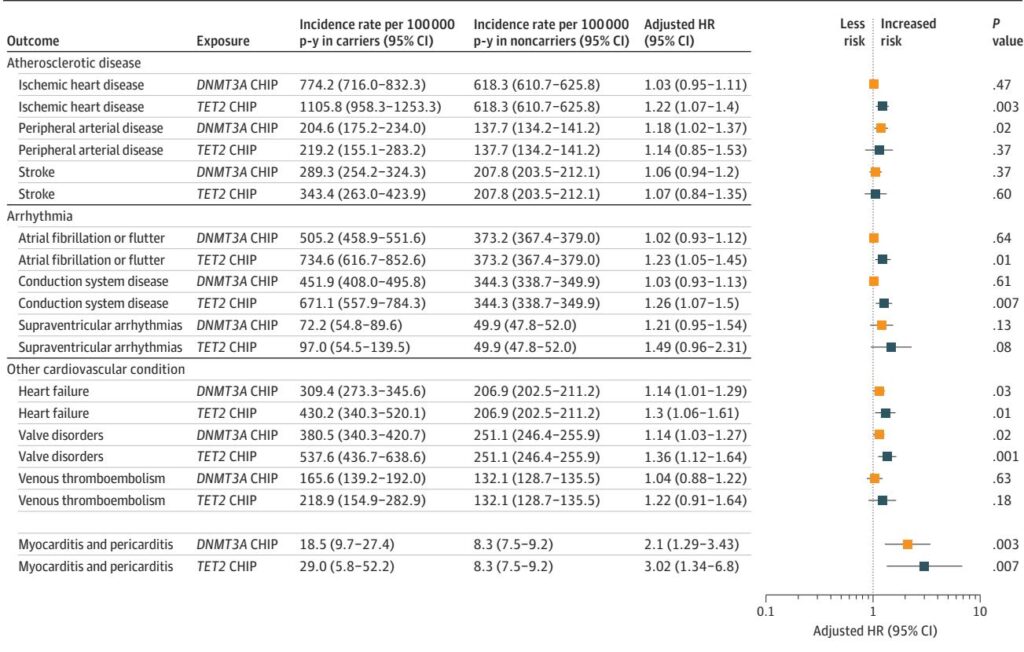
Among participants, 3.3% harbored any CHIP and 2.2% had large clones. Over follow-up, 382 individuals developed myocarditis or pericarditis. Presence of any CHIP was associated with a 1.75-fold increased risk (95% CI, 1.14–2.68; P=0.01) and large CHIP with a 2.07-fold increased risk (95% CI, 1.28–3.33; P=0.003) for the composite outcome.
Gene-specific analysis revealed DNMT3A mutations correlated strongly with pericarditis risk (HR 2.22; 95% CI, 1.17–4.21) and TET2 mutations with myocarditis (HR 3.65; 95% CI, 1.16–11.49). These associations exceeded those observed for established CHIP-related cardiovascular diseases such as coronary artery disease and heart failure. CHIP was also linked to a modestly elevated risk of noncardiac IMIDs (HR 1.27; 95% CI, 1.16–1.39), including psoriasis, rheumatoid arthritis, and vasculitis, though mediation analyses indicated these conditions explained less than 1% of the CHIP-myocarditis/pericarditis association.
Sensitivity analyses consistently supported these findings, adjusting for cancer history, cardiovascular events, and varying outcome definitions. Dose-response relationships were observed with increasing VAF and number of driver mutations correlating with greater risk.
Mechanistic Insights and Clinical Implications
Experimental models implicate CHIP, especially DNMT3A and TET2 mutations, in cardiac inflammation mediated by activation of the NLRP3 inflammasome and downstream cytokines IL-1β and IL-6. These inflammatory pathways contribute to myocarditis and pericarditis pathogenesis, supported by clinical responses to IL-1 blockade therapies in resistant cases and recurrent pericarditis.
CHIP thus represents a plausible novel mechanism driving inflammatory heart diseases beyond traditional risk factors. Targeted anti-inflammatory approaches, potentially including IL-1 inhibition, may represent promising therapeutic strategies, particularly in CHIP carriers.
Limitations
The study’s reliance on whole-exome sequencing may underestimate CHIP prevalence, especially for low VAF clones. Predominantly White participant ethnicity limits generalizability. The rarity of myocarditis and pericarditis restricted power for detailed subgroup and gene-specific analyses beyond DNMT3A and TET2. CHIP was assessed only at baseline, precluding detection of incident clonal expansions. Residual confounding cannot be fully excluded despite comprehensive adjustments. Outcome classification based on administrative codes may introduce misclassification bias.
Conclusion
In a large population-based cohort, clonal hematopoiesis emerged as a significant independent risk factor for new-onset myocarditis and pericarditis, with the strongest associations observed for DNMT3A and TET2 mutations and larger clone sizes. These findings underscore the role of somatic hematopoietic mutations in inflammatory cardiac conditions, highlighting CHIP and its inflammatory pathways as potential targets for prevention and treatment strategies. Further mechanistic and clinical trials are warranted to explore therapeutic modulation of CHIP-driven inflammation in myocarditis and pericarditis.
Reference
Schuermans A, Flynn S, Niroula A, Uddin MM, Sinnaeve P, Budts W, Conrad N, Ebert BL, Libby P, Lin AE, Weber BN, Natarajan P, Honigberg MC. Clonal Hematopoiesis and Risk of New-Onset Myocarditis and Pericarditis. JAMA Cardiol. 2025 Aug 30. doi: 10.1001/jamacardio.2025.3369 IF: 14.1 Q1 . Epub ahead of print. PMID: 40884495 IF: 14.1 Q1 .