Highlight
- ADCs have revolutionized targeted breast cancer therapy, demonstrating substantial efficacy in HER2+, HR+, and TNBC subtypes.
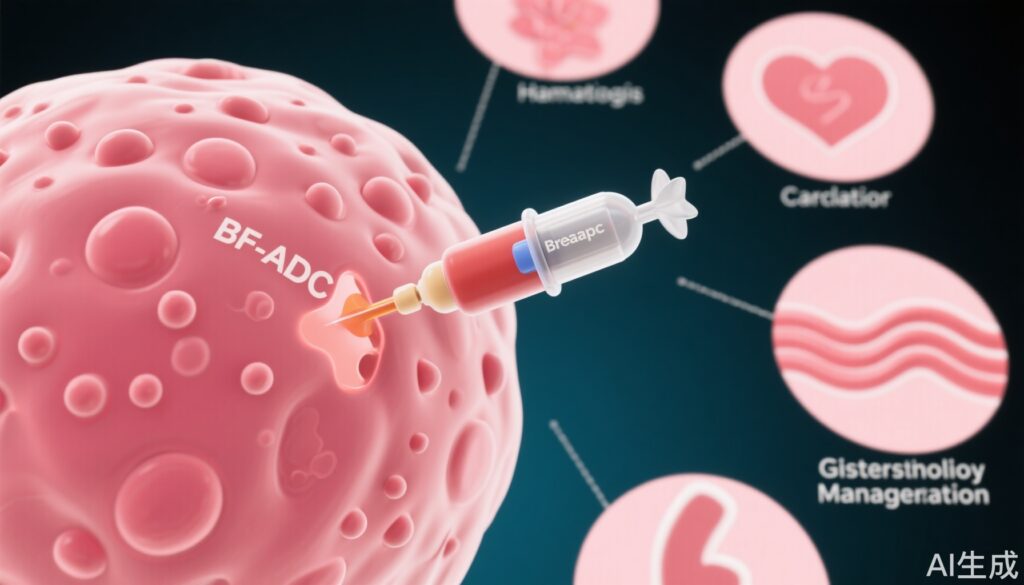
- Toxicity remains a clinical challenge, with high rates of hematologic, cardiac, and gastrointestinal adverse events necessitating vigilant monitoring and proactive management.
- Recent meta-analyses and pivotal clinical trials inform current best practices for managing ADC-related toxicities, including neutropenia, cardiotoxicity, and GI side effects.
- Ongoing research is focused on developing safer ADCs, novel payloads, and innovative linker technologies to reduce off-target effects and enhance therapeutic selectivity.
Background
Breast cancer remains the most frequently diagnosed malignancy among women worldwide, with metastatic disease accounting for a significant proportion of cancer-related mortality. While traditional chemotherapy and monoclonal antibody therapies have improved outcomes, they are often limited by nonspecific toxicity and resistance. Antibody-drug conjugates (ADCs) represent a major advance, coupling the specificity of monoclonal antibodies with the potency of cytotoxic agents, delivered via stable chemical linkers. This approach aims to maximize tumor cell kill while minimizing collateral damage to normal tissues. Several ADCs—trastuzumab emtansine (T-DM1), trastuzumab deruxtecan (T-DXd), datopotamab deruxtecan (Dato-DXd), patritumab-DXd, and sacituzumab govitecan (SG)—have achieved FDA approval for various metastatic and early-stage breast cancer indications. However, the unique toxicity profile of ADCs, distinct from their unconjugated components, presents new management challenges for clinicians.
Study Overview and Methodological Design
The clinical understanding of ADC-related efficacy and toxicity stems from pivotal randomized controlled trials (RCTs), open-label phase 1/2 studies, and meta-analyses. Notable among these are the DESTINY-Breast01, DESTINY-Breast03, and DESTINY-Breast04 trials (T-DXd), EMILIA and TH3RESA trials (T-DM1), ASCENT (SG), and the pooled analysis by Zhu et al. These studies generally enrolled patients with advanced or metastatic breast cancer, stratified by tumor subtype (HER2+, HR+, TNBC), and prior therapies. Endpoints included objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and the incidence/severity of treatment-emergent adverse events, graded per CTCAE criteria. Meta-analyses, such as Zhu et al., synthesized data across trials to provide a broader toxicity profile, reporting both all-grade and grade ≥3 adverse events, discontinuation rates, and specific toxicities by organ system.
Key Findings
Efficacy data from major trials confirm the clinical value of ADCs across breast cancer subtypes. However, toxicity rates are significant. Zhu et al. reported that 91.2% of patients experienced at least one treatment-related adverse event, with 46.1% experiencing grade ≥3 events and 13.2% discontinuing therapy due to toxicity. Hematologic toxicities are predominant: lymphopenia (53% all-grade), neutropenia (31.2% grade ≥3), anemia (up to 8.1% grade ≥3 with T-DXd), and thrombocytopenia (up to 12% severe with T-DM1). Cardiotoxicity, though less frequent, is notable for HER2-targeted ADCs, with LVEF reductions reported in up to 11.9% of patients (DESTINY-Breast04). Gastrointestinal events, including nausea, vomiting, and diarrhea, are highly prevalent—T-DXd and SG are now classified as highly emetogenic. Interstitial lung disease (ILD)/pneumonitis, particularly with T-DXd, has emerged as a serious, sometimes fatal, risk with an incidence of up to 15.8% in pooled analyses.
Mechanistic Insights and Pathophysiological Context
ADCs are designed to exploit tumor-specific antigens—HER2, Trop-2, HER3—to deliver cytotoxic agents directly to malignant cells. The monoclonal antibody component ensures specificity, while the linker controls the release of the payload (e.g., DM1, deruxtecan, SN-38) within the tumor microenvironment or upon internalization. Off-target toxicity arises from premature payload release or bystander effects, wherein cytotoxic metabolites diffuse to adjacent normal tissues. Hematologic toxicities likely result from bone marrow exposure to the payload, whereas cardiotoxicity is attributed to HER2 expression on cardiac myocytes. Gastrointestinal side effects are associated with off-target effects of highly potent payloads on rapidly dividing mucosal cells. ILD/pneumonitis is postulated to result from immune-mediated or direct cytotoxic injury to alveolar structures, with risk factors including age, prior lung disease, and cumulative ADC exposure.
Clinical Implications
Optimizing the benefit-risk ratio of ADC therapy requires proactive monitoring and tailored management of adverse events.
- Hematologic toxicities: Dose modifications, treatment holds, and selective use of granulocyte colony-stimulating factors (G-CSF) are standard for neutropenia and anemia. Thrombocytopenia management involves withholding and dose reduction.
- Cardiotoxicity: Baseline and serial cardiac monitoring (ECG, echocardiography, natriuretic peptides) are recommended. Treatment interruption or discontinuation is warranted for significant LVEF declines or heart failure.
- Gastrointestinal toxicities: For highly emetogenic agents (T-DXd, SG), prophylactic multi-agent antiemetic regimens are advised. Diarrhea is managed with loperamide and supportive care; severe cases may require dose modification or discontinuation.
- ILD/pneumonitis: Routine imaging and prompt corticosteroid initiation are critical, with permanent discontinuation for grade ≥2 events.
Guidelines such as those from the European Society of Cardiology and the National Comprehensive Cancer Network provide specific recommendations tailored to each ADC and toxicity profile. Multidisciplinary collaboration—including oncology, cardiology, pulmonary, and supportive care teams—is essential for optimal patient outcomes.
Limitations and Controversies
Despite the robust evidence base, several limitations persist. Many clinical trials exclude patients with significant comorbidities or organ dysfunction, limiting generalizability. Real-world toxicity rates may differ from trial populations. The optimal management of rare or late-onset toxicities remains unclear, and standardized protocols are evolving. The risk of ILD with T-DXd, for example, is an area of ongoing debate, with incidence varying by ethnicity and prior therapy exposure. Furthermore, the emergence of resistance mechanisms and the long-term sequelae of repeated ADC exposure are not fully understood.
Expert Commentary or Guideline Positioning
Recent consensus statements underscore the importance of individualized risk assessment and early intervention. The 2022 ESC Cardio-Oncology guidelines recommend frequent cardiac monitoring during HER2-ADC therapy. The NCCN categorizes T-DXd and SG as highly emetogenic, warranting aggressive prophylaxis. Expert opinion highlights the need for patient education regarding early symptom reporting, particularly for respiratory and GI symptoms, to facilitate timely diagnosis and management of ILD and severe diarrhea.
Conclusion
ADCs represent a paradigm shift in the management of breast cancer, offering potent efficacy with a degree of target specificity. However, their unique toxicity profile necessitates vigilant monitoring, multidisciplinary management, and ongoing patient education. Future research should focus on refining ADC design to mitigate off-target effects, developing predictive biomarkers for toxicity risk, and expanding therapeutic indications. As the ADC landscape evolves, balancing efficacy with safety remains central to maximizing patient benefit.
References
1. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610-621.
2. von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628.
3. Bardia A, Tolaney SM, Loirat D, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529-1541.
4. Pondé N, Brandão M, El-Hachem G, et al. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev. 2019;75:27-37.
5. Zhu L, Wang Z, Wang D, et al. Adverse events of antibody-drug conjugates in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2023;198(2):423-437.
6. European Society of Cardiology. 2022 ESC Guidelines on cardio-oncology. Eur Heart J. 2022;43(41):4229-4361.
7. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Antiemesis. Version 1.2024.