Background and Disease Burden
Chronic hepatitis B virus (HBV) infection remains a major global health issue, being a leading cause of hepatocellular carcinoma (HCC), the predominant form of primary liver cancer. Despite advances in antiviral therapies, the risk of HCC persists, particularly in individuals with cofactors such as chronic alcohol consumption. Alcohol abuse synergistically elevates HCC risk in HBV-infected patients through mechanisms that remain incompletely understood. Understanding the molecular interplay between alcohol and HBV pathogenesis is crucial to identifying novel therapeutic targets and improving patient outcomes.
Study Design and Methods
This study utilized an integrated preclinical approach combining genetic mouse models, cell lines, and human liver samples to elucidate the molecular mechanism by which alcohol exacerbates HBV-related hepatocarcinogenesis. Specifically, HBx-transgenic (HBx-Tg) mice—which express the HBV X protein and recapitulate HBV-driven liver tumorigenesis—were subjected to chronic ethanol feeding to model alcohol exposure. Nude mouse xenografts were employed to investigate ethanol’s effect on tumor progression.
Human liver tissues and serum from HCC patients were analyzed to correlate molecular findings with clinical outcomes. Key molecular players, including lysosomal phospholipase A2 (LPLA2), its upstream regulator activating transcription factor 4 (ATF4), and bis(monoacylglycero)phosphate (BMP) lipid species, were profiled using liquid chromatography-mass spectrometry (LC-MS) and molecular biology techniques. Functional assays in HepG2.215 and Hep3B human hepatoma cell lines assessed the oncogenic roles of LPLA2 and BMP metabolism. Gene knockdown and overexpression strategies interrogated the signaling pathways involved, with particular focus on the MAPK/ERK axis.
Key Findings
Chronic ethanol intake markedly promoted spontaneous tumor formation in HBx-Tg mice and enhanced tumor growth in nude mouse xenografts, demonstrating a clear tumor-promoting effect of alcohol in an HBV context.
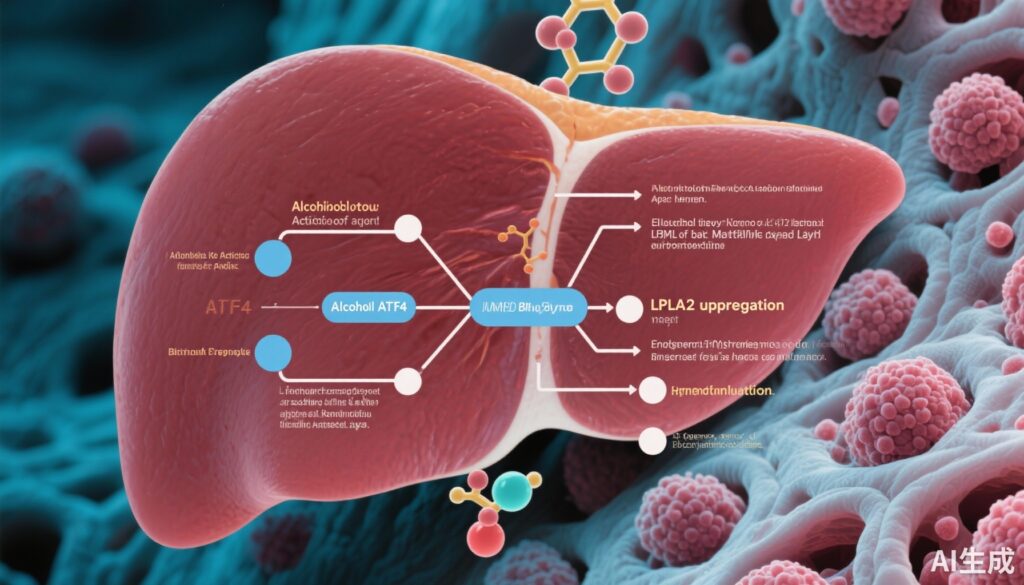
At the molecular level, LC-MS analysis revealed a significant elevation in BMP lipid species within tumors from ethanol-fed HBx-Tg mice, linking altered lipid metabolism to alcohol-mediated carcinogenesis. This increase was driven by upregulated LPLA2 expression, a lysosomal enzyme responsible for BMP lipid metabolism.
Mechanistic insights identified ATF4, a stress-responsive transcription factor, as the upstream regulator enhancing LPLA2 gene expression through direct promoter binding. Functional assays showed that overexpression of LPLA2 stimulated tumor cell proliferation in vitro and accelerated tumor growth in vivo by activating the MAPK/ERK signaling pathway. Conversely, silencing LPLA2 effectively mitigated ethanol-enhanced hepatocarcinogenesis.
Further experiments established that overexpression of BMP synthase CLN5 and BMP supplementation similarly activated MAPK/ERK signaling and promoted HCC proliferation. Importantly, CLN5 knockdown diminished the tumorigenic effects induced by LPLA2, demonstrating the critical role of BMP metabolism downstream of LPLA2.
Clinical correlation analysis uncovered that elevated LPLA2 expression in human HCC tissues is associated with poorer prognosis, underscoring its relevance as a potential biomarker.
Expert Commentary
This study provides compelling evidence for a novel molecular mechanism by which alcohol exacerbates HBV-related liver cancer, identifying the ATF4/LPLA2/BMP metabolic axis as a pivotal driver of tumorigenesis through MAPK/ERK pathway activation. The use of robust preclinical models alongside human clinical data strengthens the translational significance of these findings.
Notably, the definition of a lipid metabolic pathway closely linked to oncogenic signaling offers a fresh perspective on the interplay between metabolic dysregulation and cancer progression in HBV plus alcohol-related HCC.
Some limitations include the focus on a single molecular axis without addressing potential interactions with other pathways implicated in alcohol or HBV carcinogenesis. Additionally, while the prognosis association of LPLA2 was demonstrated, its utility as a predictive biomarker awaits further validation in large prospective cohorts.
Conclusion and Implications
This work elucidates critical molecular underpinnings of how chronic alcohol consumption amplifies HBV-induced hepatocellular carcinoma via activation of the ATF4/LPLA2-mediated BMP metabolism, which fuels MAPK/ERK signaling and tumor cell proliferation. Importantly, LPLA2 emerges as a novel therapeutic target and prognostic marker in HBV-HCC.
The demonstration that targeting LPLA2 or modulating BMP metabolism significantly inhibits HBV-HCC progression in preclinical models supports the rationale for developing targeted therapies aimed at this lipid metabolic axis.
Future research should expand on these findings to explore combinational interventions targeting metabolic and signaling pathways, as well as clinical trials to evaluate LPLA2 inhibitors or BMP metabolic modulators in patients with HBV-related liver cancer, particularly those with concurrent alcohol use.
Funding and Registration
The original research was published by Zhou et al. in the Journal of Hepatology (2025). Specific funding sources were not disclosed in the summary provided.
References
Zhou H, Ba J, Xiao C, Liu H, Jiang J, Guo Y, Wu B. Alcohol activates ATF4/LPLA2-mediated BMP metabolism to enhance HBV-induced hepatocellular carcinogenesis. J Hepatol. 2025 Aug 28:S0168-8278(25)02458-4. doi: 10.1016/j.jhep.2025.08.022. Epub ahead of print. PMID: 40885211.
Additional relevant literature:
1. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
2. Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7(8):599-612.
3. Lee YH, et al. Integrative analysis reveals prognostic significance and molecular subtype diversity in hepatocellular carcinoma. Hepatology. 2018;68(3):1142-1155.