Highlight
This randomized clinical trial evaluates a novel radiotherapy approach—modified target delineation guided by multimodal magnetic resonance imaging (MRI) and white matter tractography combined with moderately hypofractionated simultaneous boost intensity-modulated radiotherapy (HSIB-IMRT)—in high-grade glioma (HGG). The study found that this technique significantly reduces clinical target volumes without compromising progression-free survival (PFS), overall survival (OS), or increasing recurrence outside irradiated areas. Safety profiles were comparable to standard treatment, underscoring the potential for personalized, volume-sparing radiotherapy to mitigate neurotoxicity in HGG management.
Study Background and Disease Burden
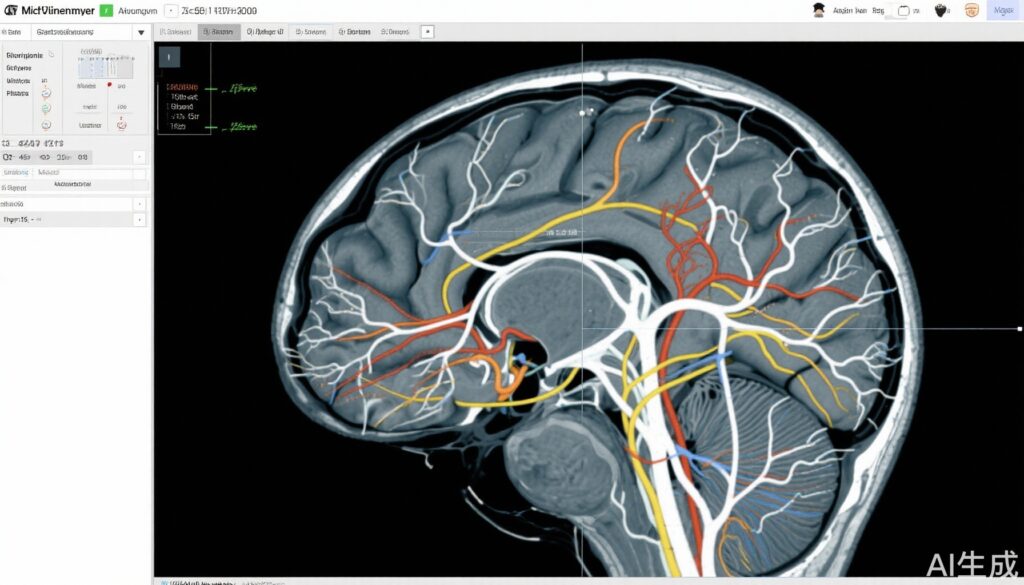
High-grade gliomas (HGG), including glioblastoma multiforme and anaplastic gliomas, remain among the most aggressive and lethal primary brain tumors. Despite multimodal treatment combining maximal safe surgical resection, radiotherapy, and temozolomide chemotherapy, median survival rarely exceeds two years. Radiotherapy is a cornerstone of management; however, optimal irradiation target volumes and dosing schedules remain areas of active research. Traditionally, broad clinical target volumes (CTVs) are delineated to encompass presumed microscopic tumor infiltration zones, frequently leading to exposure of healthy brain tissue and increased risk of neurotoxicity. With advances in neuroimaging, including multimodal MRI and diffusion tensor imaging delineating white matter tracts, there is promise to more precisely map tumor spread pathways, enabling more selective radiotherapy target delineation.
Moreover, fractionation schedules have important implications for efficacy and toxicity. Moderately hypofractionated radiotherapy, delivering slightly larger doses per fraction with simultaneous boost techniques, may improve tumor control without additional toxicity. There is, therefore, an unmet need to refine both irradiation volumes and fractionation schemes to enhance clinical outcomes and quality of life for HGG patients.
Study Design
This was a single-center, prospective, open-label, randomized clinical trial conducted at a Chinese medical center. A total of 154 patients aged between 18 and 70 years with histologically confirmed, newly diagnosed high-grade glioma were enrolled from January 2018 to August 2022, with follow-up completed in June 2024.
Patients were randomized into two arms:
- Experimental Arm: Target delineation was modified and guided by multimodal MRI techniques (including contrast-enhanced T1-weighted MRI, T2/FLAIR sequences) integrated with white matter tractography to identify tumor infiltration pathways. This was combined with moderately hypofractionated simultaneous boost intensity-modulated radiotherapy (HSIB-IMRT), delivering a higher boost dose to high-risk areas within the gross tumor volume while using a reduced overall target volume.
- Standard Arm: Patients received standard intensity-modulated radiotherapy (IMRT) based on established guideline-recommended clinical target volumes without integration of advanced imaging-guided modifications.
Both arms concurrently received temozolomide chemotherapy during radiotherapy and adjuvant temozolomide thereafter, following Stupp protocol principles.
The primary endpoint was progression-free survival (PFS), defined as the time from randomization to disease progression or death. Secondary endpoints included overall survival (OS) and safety, focusing on treatment-related adverse events.
Key Findings
Among the 154 enrolled patients, median follow-up was 22 months (range 4–76 months), with 96 deaths observed by the study conclusion. Baseline characteristics including age (median 51.5 years) and sex distribution (55.2% male) were comparable between arms.
Progression-Free Survival: The median PFS was 15.5 months (95% CI, 11.7–19.3 months) in the experimental arm vs 13.5 months (95% CI, 8.7–18.3 months) in the standard arm, with no statistically significant difference (P = .89).
Overall Survival: Median OS was 27.0 months (95% CI, 13.9–40.1 months) in the experimental arm and 21.0 months (95% CI, 18.0–24.0 months) in the standard arm. This difference did not reach statistical significance (P = .24), but numerically favored the experimental approach.
Clinical Target Volume: The median CTVs in the experimental group were significantly smaller than those in the standard group (CTV1 median 116.7 cm3 vs 225.0 cm3, P < .001; CTV2 median 174.4 cm3). The reduction in irradiated brain volume suggests a potential for decreased radiation-associated neurotoxicity.
Recurrence Patterns: Rates of tumor recurrence within, outside, or multicentric relative to the irradiated target volumes were comparable between the two groups, indicating no increased risk of marginal or out-of-field failure with smaller CTVs guided by advanced imaging.
Safety: Grade 3 or 4 adverse events related to treatment occurred in 5.3% of patients in the experimental arm and 3.8% in the standard arm (P = .72), demonstrating similar tolerability.
Expert Commentary
This trial represents a significant advancement toward personalized radiotherapy in HGG. The integration of multimodal MRI and white matter tractography for target delineation improves anatomical precision in identifying potential tumor spread pathways, challenging conventional larger margin concepts. Moderately hypofractionated simultaneous boost IMRT offers a tailored dose escalation within high-risk tumor regions, theoretically improving tumor control.
While the primary endpoints of PFS and OS did not show statistically significant differences, the experimental approach achieved comparable oncologic outcomes with markedly reduced irradiation volumes. This could translate into meaningful long-term clinical benefits by minimizing neurocognitive decline and radiation-induced toxicity, which are major concerns in brain tumor survivors.
Limitations include the single-center setting and relatively modest sample size, which may limit generalizability. Future multicenter studies with larger cohorts and longer follow-up are needed to confirm these results and assess neurocognitive and quality-of-life endpoints. Additionally, ongoing advances in imaging biomarkers may further refine personalized target delineation.
Conclusion
Modified target delineation guided by multimodal MRI and white matter tracts combined with moderately hypofractionated simultaneous boost IMRT offers a promising strategy for treating newly diagnosed high-grade glioma. By significantly reducing clinical target volumes without compromising survival or increasing recurrence risk, this approach supports a shift towards more personalized, volume-sparing radiotherapy protocols. Such innovations may ultimately improve long-term functional outcomes and quality of life for patients with this devastating disease.
These findings warrant incorporation into future clinical trial designs and consideration by radiation oncology guidelines aiming to optimize HGG management.
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. doi:10.1056/NEJMoa043330
- Weller M, van den Bent M, Tonn JC, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170-186. doi:10.1038/s41571-020-00447-z
- Alexander BM, Ba S, Sampson JH, et al. Tumor treatment fields in malignant gliomas: past, present, and future. Neuro Oncol. 2020;22(2):190-199. doi:10.1093/neuonc/noz161
- Yang W, Yan Q, Zhang A, et al. Modified Target Delineation and Moderately Hypofractionated Radiotherapy for High-Grade Glioma: A Randomized Clinical Trial. JAMA Netw Open. 2025;8(7):e2523053. doi:10.1001/jamanetworkopen.2025.23053