Study Background and Disease Burden
Locally advanced rectal cancer remains a therapeutic challenge due to its propensity for local recurrence and the morbidity associated with standard surgical approaches such as total mesorectal excision (TME). Although TME is the gold standard for curative intent, it can result in significant functional impairments and reduced quality of life. Recently, total neoadjuvant therapy (TNT)—which integrates chemoradiotherapy followed by consolidation chemotherapy prior to surgery—has demonstrated improved pathological complete response (pCR) rates and enhanced disease-free survival. However, the possibility of organ preservation through a non-operative management strategy called “watch and wait” in patients achieving a clinical complete response (cCR) post-TNT has garnered considerable interest but requires further prospective validation.
Study Design
The CAO/ARO/AIO-16 trial was an open-label, multicentre, single-arm phase 2 study conducted at four German centers. Ninety-three patients aged 18 or older with histologically confirmed rectal adenocarcinoma (cT1-2N1-2 or cT3a-dN0/N1-2) located within 12 cm of the anal verge and no distant metastases were enrolled. Treatment consisted of concurrent chemoradiotherapy: 50.4 Gy delivered over 28 fractions combined with continuous infusion fluorouracil and intermittent oxaliplatin infusions. This was followed by three cycles of consolidation FOLFOX (fluorouracil, oxaliplatin, and leucovorin). Tumor response was assessed at day 106 and for near cCRs, a second reassessment occurred on day 196. Patients with cCR entered watch-and-wait surveillance; those with near cCRs underwent reassessment and potential local excision if feasible, while poor responders proceeded to immediate TME.
Key Findings
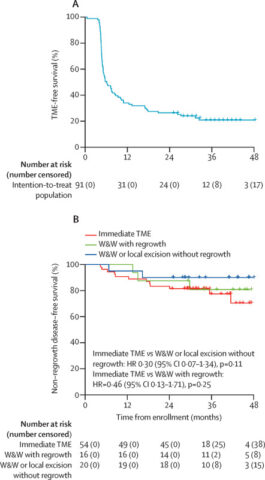
Among 91 patients who started chemoradiotherapy, 88 proceeded to consolidation chemotherapy and underwent response assessment. At first evaluation (day 106), 15% achieved cCR and were placed on watch and wait, 38% were near cCR and reassessed at day 196, and 48% received immediate TME.
During the second assessment, 64% of near cCR patients converted to cCR, with two undergoing local excision with pathological complete response and watch and wait, and the remainder undergoing TME. Thus, the overall clinical complete response rate reached 37% (34/91) after TNT.
Toxicity was notable but manageable. Grade 3 or 4 adverse events occurred in 36% of patients during TNT; diarrhoea and infections were the most frequent grade 3 toxicities during chemoradiotherapy, while leucopenia and neutropenia predominated during consolidation chemotherapy. One patient died due to COVID-19 pneumonia. During follow-up, 21% experienced grade 3 or 4 adverse events.
Expert Commentary
The CAO/ARO/AIO-16 trial provides compelling evidence that upfront chemoradiotherapy followed by consolidation chemotherapy can yield a high clinical complete response rate, enabling a substantial subset of patients to avoid radical surgery through an organ preservation strategy. This addresses one of the critical unmet needs in rectal cancer management: improving quality of life without compromising oncological safety. The watch-and-wait approach, backed by rigorous assessment protocols including digital rectal examination, endoscopy, and pelvic MRI, offers a paradigm shift that moves beyond traditional surgical dogma.
However, patient selection and longitudinal monitoring are paramount. The potential for tumor regrowth necessitates close surveillance and timely salvage therapy. Additionally, the applicability of these findings to broader populations and diverse healthcare settings warrants further study.
Conclusion
The CAO/ARO/AIO-16 phase 2 study demonstrates that total neoadjuvant therapy combined with a watch-and-wait strategy achieves a notable clinical complete response rate of 37% in locally advanced rectal cancer patients while maintaining an acceptable safety profile. This approach provides a promising organ-preserving alternative to immediate TME surgery, potentially reducing morbidity and improving patient-centered outcomes. Future research should focus on refining predictive markers for response, optimizing assessment timing, and confirming long-term oncological outcomes.
References
Gani C, Fokas E, Polat B, et al. Organ preservation after total neoadjuvant therapy for locally advanced rectal cancer (CAO/ARO/AIO-16): an open-label, multicentre, single-arm, phase 2 trial. Lancet Gastroenterol Hepatol. 2025 Jun;10(6):562-572. doi: 10.1016/S2468-1253(25)00049-4 IF: 38.6 Q1 . PMID: 40347958 IF: 38.6 Q1 .