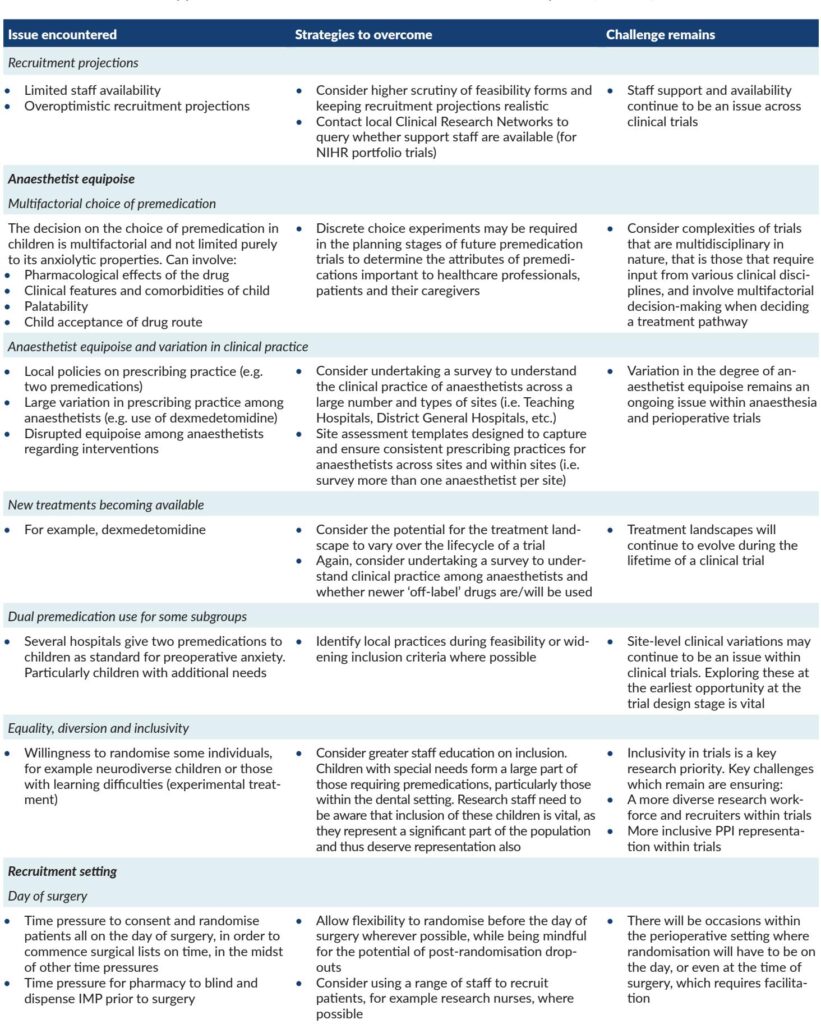
Background
Anxiety in children before undergoing general anaesthesia (GA) is prevalent, with approximately 50% of pediatric patients exhibiting distress behaviors. Such preoperative anxiety can adversely affect the anaesthetic process, potentially leading to non-compliance, unsuccessful inductions, increased postoperative pain, agitation, behavioral disturbances, and sleep disruptions. Midazolam, a benzodiazepine, is the current standard premedication used to mitigate this anxiety. However, midazolam carries risks including respiratory depression, variable pharmacologic responses, and paradoxical reactions such as agitation rather than calming effects, especially notable in children with comorbidities or additional needs. Melatonin, a naturally occurring hormone with sedative and anxiolytic properties, has emerged as a candidate alternative due to its favorable safety profile. Prior studies involving melatonin in adults suggest comparable anxiolytic effectiveness to midazolam, but data in pediatric populations remain conflicting, with previous trials limited by small sample sizes and heterogeneous patient groups. The Melatonin for Anxiety prior to General anaesthesia In Children (MAGIC) trial was designed to rigorously evaluate whether melatonin is non-inferior to midazolam in reducing anxiety in anxious children scheduled for elective surgery under GA within the United Kingdom National Health Service (NHS) context.
Study Design
MAGIC was a multicenter, parallel-group, randomized controlled, double-blind, non-inferiority trial conducted across 20 NHS trusts in the UK. The trial enrolled children aged 3 to 14 years who exhibited significant preoperative anxiety and were scheduled for elective day-case surgery under general anaesthesia, including dental, ENT, ophthalmic, and other surgical specialties.
Participants were randomized in a 1:1 ratio to receive either standard care with oral midazolam or oral melatonin as premedication. The primary efficacy outcome was preoperative anxiety measured by the modified Yale Preoperative Anxiety Scale Short Form (mYPAS-SF) at three critical time points: start of transfer to theatre, entry into the anaesthetic room, and induction of anaesthesia. The trial defined a non-inferiority margin of 4.3 points on the mYPAS-SF scale.
Secondary outcomes included safety profiles (adverse events, serious adverse events), recovery metrics (turnaround time, emergence delirium assessed via the PAED scale), postoperative pain (revised Faces Pain Scale – Observer and Participant reported), analgesic requirements, behavioral changes at 14 days post surgery (using PHBQ-AS), and cost-effectiveness analysis over a 14-day follow-up.
An integrated qualitative substudy explored barriers to recruitment and acceptability of the interventions among stakeholders, including clinicians, caregivers, and children. Additionally, a health economic evaluation assessed costs related to each premedication approach.
Key Findings
Recruitment challenges, compounded by the COVID-19 pandemic, Brexit-related delays, research pharmacy operational constraints, and anaesthetist equipoise variations, led to premature trial closure after enrolling 110 children (55 per arm) against a planned sample size of 624.
Despite early termination, statistical analyses were conducted using both intention-to-treat (ITT) and per-protocol (PP) populations. Results showed that midazolam significantly outperformed melatonin in reducing preoperative anxiety. Adjusted mean differences (mYPAS-SF score) were 13.1 (95% CI 3.7 to 22.4) in the ITT and 12.9 (95% CI 3.1 to 22.6) in the PP analysis, favoring midazolam. The upper confidence bounds exceeded the pre-specified non-inferiority margin, leading to the conclusion that melatonin is not non-inferior but inferior to midazolam in this context. The magnitude of difference was deemed clinically meaningful.
Secondary outcomes, including rates of anaesthetic failure, recovery times, postoperative pain scores, analgesic use, emergence delirium, and 14-day behavioral changes, showed no statistically significant differences between groups. Adverse events were slightly more frequent in the midazolam group (26%) versus melatonin (18%), though no serious adverse events were reported in either arm.
Health economic analysis indicated marginally lower costs over 14 days postoperatively for the melatonin group (mean difference –£46.20), but uncertainty in cost-effectiveness was considerable, and the lower efficacy calls into question the practical value of these findings.
Qualitative interviews revealed complex factors influencing recruitment and premedication choice, including caregiver stress, institutional pharmacy constraints, anaesthetists’ preference for predictability, and multifactorial considerations beyond pure anxiolytic efficacy—such as medication palatability, route of administration, and side effect profiles.
Expert Commentary
The MAGIC trial uniquely targeted anxious children at baseline, enhancing clinical relevance and distinguishing its findings from prior smaller, mixed-population studies where melatonin’s efficacy remained equivocal. The robust double-blind, randomized design and multicenter recruitment support result validity within the NHS setting.
Nevertheless, the substantial under-recruitment imposes limitations, increasing the risk of bias and constraining secondary outcome interpretations. Early trial cessation due to systemic and logistical challenges—exacerbated by the COVID-19 pandemic and regulatory changes—reflects persistent difficulties in pediatric perioperative research.
While midazolam’s sedative side effects and rare adverse profiles remain concerns, this study reinforces its primacy in managing preoperative anxiety in children, rejecting melatonin as a suitable replacement. Emerging alternatives such as intranasal dexmedetomidine, with promising tolerability and efficacy, warrant further head-to-head evaluations against midazolam.
The complex landscape of anxiolytic premedication—encompassing efficacy, safety, administration, caregiver preference, and institutional logistics—calls for multidimensional assessment methods in future trials, including discrete choice experiments to elucidate stakeholder priorities.
Conclusion
In children with significant preoperative anxiety undergoing elective surgery under general anaesthesia, midazolam remains more effective than melatonin in reducing anxiety prior to induction. Despite melatonin’s favorable safety profile and potential cost benefits, it cannot be recommended as a substitute premedication based on current evidence.
The MAGIC trial underscores the challenges inherent in perioperative pediatric research, from recruitment to evolving clinical practices. Ongoing research is needed to identify or repurpose anxiolytic agents with efficacy comparable to midazolam but improved safety and recovery profiles. Additionally, integrating stakeholder preferences is vital to developing patient-centered premedication strategies.
References
Deery C, Bolt R, Papaioannou D, Wilson M, Hyslop M, Herbert E, Totton N, Marshman Z, Young T, Kettle J, Albadri S, Atkins S, Biggs K, Clarkson J, Evans C, Flight L, Gath J, Gilchrist F, Hutchence K, Ireland N, Loban A, Norrington A, Paton H, Ray J, Rodd H, Sheldon E, Simmonds R, Vernazza C. Melatonin versus midazolam in the premedication of anxious children attending for elective surgery under general anaesthesia: the MAGIC non-inferiority RCT. Health Technol Assess. 2025 Jul;29(29):1-25. doi: 10.3310/CWKF1987. PMID: 40622250; PMCID: PMC12278375.