Highlight
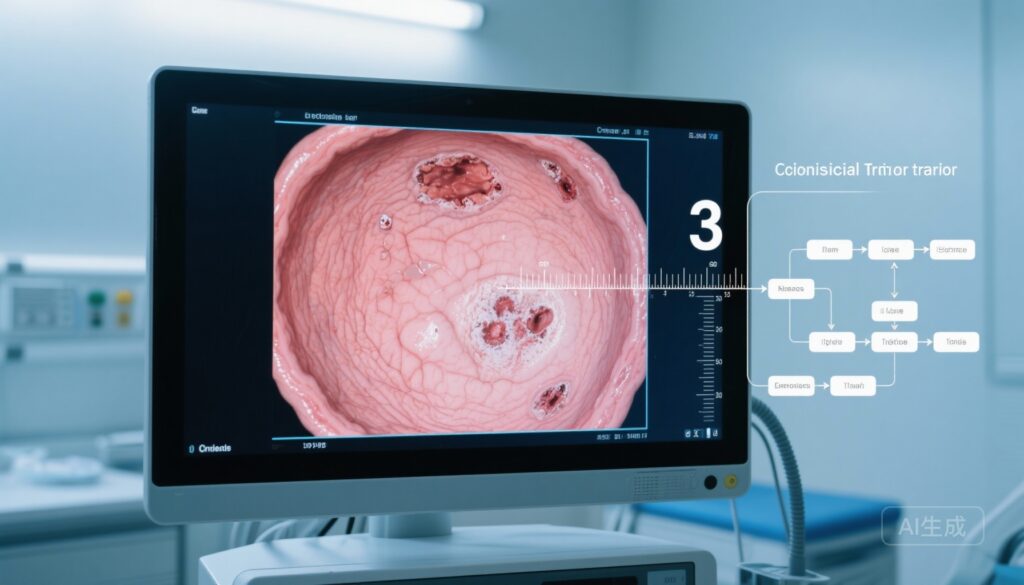
- EASE‑CD is a continuous 0–100 endoscopic index emphasizing ulcer burden (presence, size, depth, and proportion of ulcerated surface) with straightforward item definitions.
- Developed from central reader assessments of paired ileocolonoscopy videos from two randomized controlled trials, EASE‑CD demonstrated substantial inter‑rater reliability (ICC 0.798) and large responsiveness to treatment (WinP 0.769).
- Internally and externally validated: optimism‑adjusted R2 ≈0.55 and excellent calibration (slopes ~0.98–0.99); a 10‑point reduction shows clinically meaningful sensitivity/specificity for conventional endoscopic response.
Background
Accurate, reliable, and responsive endoscopic indices are essential for measuring mucosal disease activity and treatment effects in Crohn’s disease (CD). Historically, indexes such as the Crohn’s Disease Endoscopic Index of Severity (CDEIS) and the Simple Endoscopic Score for Crohn’s Disease (SES‑CD) have been used in clinical trials and practice. However, these scores have limitations: complex or ambiguous item definitions, variable interobserver reliability, and limited evidence‑based thresholds that map to clinically meaningful treatment effects. Regulatory and therapeutic decision‑making increasingly demand reproducible endoscopic endpoints that reflect ulcer burden, because mucosal healing and ulcer clearance are linked to improved long‑term outcomes in CD.
Study design
Ma and colleagues performed a multiphase development and validation study using centrally read paired baseline and post‑induction ileocolonoscopy videos from two randomized controlled trials: a phase 3b adalimumab trial (NCT00348283) and a phase 2 risankizumab trial (NCT02031276). Videos were assessed by multiple central readers masked to clinical data and treatment assignment. Candidate items with face validity were selected using modified RAND/UCLA appropriateness methods. Key methodological points:
- Datasets: 112 paired ileocolonoscopy videos (adalimumab trial) used for index development, and 99 paired videos (risankizumab trial) for external validation.
- Reading strategy: four independent, blinded central readers rated videos; a subset of 44 post‑treatment videos were re‑read to estimate inter‑rater reliability in development.
- Selection criteria for items: acceptable inter‑rater reliability (ICC ≥0.41) and responsiveness (win probability [WinP] ≥0.56).
- Model building: multivariable regression with internal bootstrap validation; external validation performed on the independent risankizumab trial dataset.
Key findings and results
The investigators evaluated a panel of candidate items drawn from SES‑CD and CDEIS as well as exploratory lesion‑focused items. Ten items met pre‑specified ICC and WinP thresholds and entered model consideration. The final Endoscopic ulcer Activity Score for Evaluating CD (EASE‑CD) comprises three core components, each averaged across visualized ileocolonic segments:
- Ulcer presence and size on an ordinal scale: 0 = none, 1 = 20 mm.
- Deep ulceration: dichotomous 0 = absent, 1 = present.
- Proportion of ulcerated surface expressed continuously from 0 to 1 (fractional surface involvement).
These elements are combined into a 0–100 total score, with higher values indicating greater endoscopic activity.
Model performance
- Internal validation (development dataset): R2 = 0.608; optimism‑adjusted R2 = 0.554; calibration slope 0.983—indicating good explanatory power and near‑ideal calibration.
- External validation (risankizumab dataset): adjusted R2 = 0.565; calibration slope 0.997—demonstrating reproducibility across trials.
- Inter‑rater reliability in external validation: ICC 0.798 (95% CI 0.711–0.836), classified as substantial agreement.
- Responsiveness: WinP = 0.769 (95% CI 0.658–0.851; p < 0.001) in external validation, indicating that patients randomized to active treatment were substantially more likely to have improved EASE‑CD scores than those randomized to placebo.
- Clinical thresholds: a 10‑point reduction in EASE‑CD showed 82.4% specificity (95% CI 78.6–86.0) and 69.9% sensitivity (63.3–76.4) for endoscopic response defined as ≥50% reduction in SES‑CD or CDEIS. Ulcer‑free endoscopic remission corresponds to an EASE‑CD score of 0.
Comparison with existing indices
EASE‑CD emphasizes ulcer characteristics (size, depth, and proportional ulcerated surface), whereas SES‑CD and CDEIS include multiple features such as affected surface, number of ulcers, presence of ulcers, stenosis, and more. EASE‑CD’s parsimonious, ulcer‑focused structure and continuous scale appear to retain strong explanatory power while simplifying scoring and improving inter‑rater reproducibility. Its explicit, ordinal ulcer size categories (20 mm) and a continuous fractional ulcerated surface reduce ambiguity in interpretation that has undermined reproducibility of prior scores.
Expert commentary and clinical implications
The development of EASE‑CD addresses several unmet needs in CD outcome measurement. First, by giving ulcers—biologically central lesions in CD—a central quantitative role, the score aligns the endoscopic instrument with a pathophysiological target most closely tied to inflammation, risk of complications, and treatment aims. Second, the continuous 0–100 scale facilitates statistical power in trials while permitting clinically relevant cutoffs (for response and remission) to be derived and validated. Third, the substantial inter‑rater reliability observed with central readers suggests that EASE‑CD may be well‑suited for blinded, centrally read trial endpoints where consistency matters most.
For clinicians and trialists, EASE‑CD offers several potential advantages:
- Improved responsiveness may reduce sample size needs for trials that target ulcer healing.
- Clear item definitions (ulcer size categories, deep ulceration, and quantitative ulcerated surface) facilitate standardized training and quality control across centers.
- The mapping of a 0 score to ulcer‑free mucosa provides an intuitive definition of endoscopic remission.
Limitations and considerations
There are important considerations before broad adoption of EASE‑CD in routine clinical practice and regulatory settings:
- Development and validation used centrally read video datasets from patients enrolled in biologic trials. Generalizability to community practice, unsedated or incomplete colonoscopies, small‑bowel disease beyond the reach of ileocolonoscopy, and pediatric populations remains to be established.
- The final model averages item scores across visualized segments; cases with incomplete visualization or isolated small‑bowel lesions not reached by ileocolonoscopy may under‑represent true disease burden.
- Although EASE‑CD correlated with conventional indices (SES‑CD/CDEIS), direct associations with long‑term clinical outcomes (hospitalization, surgery, steroid‑free remission) require prospective confirmation—ideally in trials with clinical endpoints or longitudinal real‑world cohorts.
- Operational considerations remain: central reading resources, reader training, and use of still‑images versus full video review may influence performance characteristics.
Future directions
Key next steps to consolidate EASE‑CD’s role include:
- Validation in broader clinical and demographic contexts (community cohorts, pediatrics, post‑operative Crohn’s, and small‑bowel‑predominant disease using balloon enteroscopy or cross‑sectional imaging correlation).
- Head‑to‑head comparisons of EASE‑CD against SES‑CD and CDEIS in prospective trials to assess sample size implications, sensitivity to change across different therapeutic mechanisms, and predictive validity for long‑term outcomes.
- Operational studies to standardize video capture quality, reader training modules, and automated or semi‑automated image analysis leveraging artificial intelligence to estimate ulcer size and proportion of ulcerated surface.
- Regulatory engagement to consider EASE‑CD as a candidate endoscopic endpoint for drug approval and label claims, supported by outcome linkage data.
Conclusion
EASE‑CD is a pragmatic, evidence‑based endoscopic index that prioritizes ulcer burden using defined ordinal and continuous measures. It demonstrated strong reliability, responsiveness, and calibration in internal and external validation using two randomized controlled trial datasets. EASE‑CD has the potential to improve trial endpoint precision, aid interpretation of mucosal healing, and streamline central reading workflows. Further validation across broader clinical settings and correlation with long‑term patient‑centered outcomes will be essential to establish its role in clinical practice and regulatory decision‑making.
Funding and trial registration
Funding: None reported in the primary manuscript. ClinicalTrials.gov identifiers: NCT00348283 (phase 3b adalimumab trial) and NCT02031276 (phase 2 risankizumab trial).
References
Ma C, Khanna R, Maguire BR, et al. Development and validation of a novel Endoscopic ulcer Activity Score for Evaluation of Crohn’s Disease (EASE‑CD) using data from two randomised controlled trials. Lancet Gastroenterol Hepatol. 2025 Aug;10(8):746‑756. doi:10.1016/S2468‑1253(25)00093‑7.
Turner D, Ricciuto A, Lewis A, et al. STRIDE‑II: An update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease initiative of the International Organization for the Study of Inflammatory Bowel Disease (IOIBD): determining therapeutic goals for treat‑to‑target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi:10.1053/j.gastro.2020.12.031.