Highlight
- A manganese sulfide (MS) nanoplatform loaded with proton pump inhibitor (PPI) enables real-time magnetic resonance imaging (MRI) of tumor acidosis.
- The nanoplatform releases Mn2+, H2S, and PPI in acidic tumor lysosomes, inducing tumor acidosis and enhancing MRI contrast.
- Tumor acidosis correlates positively with tumor regression, enabling precognition of therapeutic efficacy much earlier than conventional structural imaging.
- Tumor acidosis induced by the nanoplatform also unexpectedly suppresses tumor metastasis, highlighting potential therapeutic benefits.
Study Background and Disease Burden
Cancer remains a major cause of morbidity and mortality worldwide, with tumor heterogeneity posing a critical challenge to timely assessment of therapeutic response. Conventional methods commonly rely on measuring anatomical or volumetric changes in tumor mass, which often require prolonged durations to manifest. This delay can result in lost opportunities to modify treatment regimens before disease progression ensues. Moreover, the acidic microenvironment of tumors is an established hallmark linked to tumor progression, invasiveness, and therapy resistance. Proton pumps and altered metabolism contribute to extracellular acidification, yet noninvasive real-time assessment of tumor acidosis remains technically challenging. Therefore, there is an unmet clinical need for innovative approaches that can dynamically map tumor acidity and provide early insights into therapeutic efficacy, allowing timely and individualized treatment adjustments.
Study Design
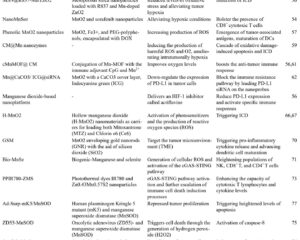
This study introduces a rationally designed manganese sulfide-based nanoplatform (MSP) loaded with a proton pump inhibitor (PPI) intended for theranostic application in cancer. MSP synthesis was accomplished via a two-step chemical method involving manganese acetylacetonate and thioacetamide in ethylene glycol, producing spheroidal manganese sulfide nanoparticles of approximately 120 nm diameter. The nanoplatform’s physicochemical properties including morphology, crystallinity, surface chemistry, and pH-responsive degradation were characterized using transmission electron microscopy (TEM), high-resolution TEM, X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS).
The functional components released by MSP—Mn2+, H2S, and PPI—were quantified under varying pH conditions to simulate the acidic milieu of tumor lysosomes. The verification of H2S evolution was performed using AgNO3 precipitation assays, and reactive oxygen species production through Fenton-like Mn2+ catalysis was evaluated by colorimetric assays. MRI relaxivity (r1) measurements were conducted to determine the pH-dependent MRI signal enhancement by MSP.
The nanoplatform was further evaluated in vitro to modulate tumor acidosis by varying PPI loading ratios (10%, 25%, 44%) and assessing the resultant effects on tumor cell lysosomal pH, glucose metabolism, and lactate production. Correlations between induced tumor acidosis, nanoplatform degradation, and MRI signal intensities were systematically analyzed. Additionally, the impact of tumor acidosis on tumor growth regression and metastatic potential were investigated.
Key Findings
The synthesized manganese sulfide nanoparticles exhibited uniform spheroidal morphology with high crystallinity consistent with MnS (002) planes. XPS confirmed the presence of Mn2+ and sulfide ions, supporting successful synthesis. Acid-triggered degradation studies demonstrated increased MSP decomposition at lower pH (pH 4.5) with maximal efficiency (79.5%), releasing Mn2+, PPI, and H2S. This degradation was accompanied by visible bubble formation and a characteristic H2S odor, and black Ag2S precipitate confirmed H2S generation.
Released Mn2+ catalyzed the production of hydroxyl radicals (•OH) via Fenton-like reactions, confirmed by decolorization and colorimetric assays, indicating potential cytotoxic effects contributing to antitumor activity. Importantly, MRI relaxivity (r1) was significantly enhanced in acidic conditions, showing a clear pH- and dose-dependent increase in T1-weighted MRI signal, with the highest relaxivity at pH 4.5 (8.243 mM−1 s−1) and lowest at neutral pH (1.628 mM−1 s−1).
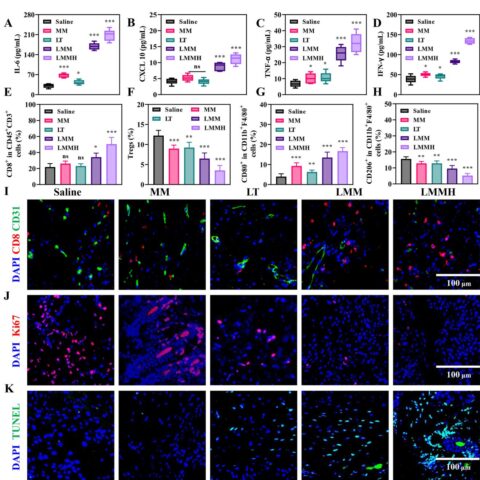
Nanoplatforms with varying PPI loadings successfully induced graded tumor acidosis by inhibiting V-ATPase pumps and increasing intracellular H+ concentration. Released H2S intensified tumor glucose consumption, yielding augmented lactic acid and reinforcing acidosis, which in turn accelerated MSP degradation and MRI signal enhancement in a positive feedback loop. Crucially, the degree of tumor acidosis correlated positively with tumor regression metrics, enabling real-time prediction of therapeutic efficacy prior to conventional structural changes.
Unexpectedly, increased tumor acidosis also suppressed metastatic dissemination, providing an additional therapeutic advantage. The nanoplatform therefore bridges diagnostics and therapeutics by simultaneously monitoring tumor microenvironment acidity and modulating tumor physiology.
Expert Commentary
This innovative manganese sulfide-based nanoplatform represents a significant advancement in the field of tumor microenvironment imaging and therapy monitoring. The capacity for real-time, noninvasive MRI of tumor acidosis provides clinicians with a dynamic biomarker reflecting tumor metabolic status and response to treatment much earlier than traditional size-based criteria.
Mechanistically, leveraging tumor lysosomal acidification to trigger compound release and induce a metabolic feedback loop exemplifies precision nanomedicine design. Additionally, the ability of H2S to reshape tumor metabolism and the Fenton-like activity of Mn2+ to generate cytotoxic radicals offers multimodal therapeutic effects inherent to the nanoplatform.
Limitations include the need for comprehensive in vivo validation, long-term safety profiling, and evaluation across diverse tumor models to confirm generalizability. Furthermore, translation into clinical practice will require scaling synthesis and regulatory pathway navigation. Nonetheless, integrating pH-responsive MRI contrast and therapeutic release within a single nanostructure could transform real-time cancer treatment monitoring and adaptive therapy strategies.
Conclusion
The manganese sulfide nanoplatform loaded with proton pump inhibitor presents a novel theranostic tool enabling real-time MRI visualization of tumor acidosis. By exploiting tumor lysosomal acidity to release therapeutic agents and enhance MRI signal, this platform offers early prediction of treatment efficacy, potentially allowing clinicians to tailor interventions dynamically. The positive correlation between tumor acidosis measured by MRI and tumor regression underscores this approach’s clinical utility. Moreover, the observed metastasis suppression highlights additional therapeutic potential. Future clinical translation may optimize cancer management by integrating timely diagnostic feedback with targeted metabolic modulation.
References
Mu M, Zhang M, Liu J, Ren K, Liu H, Yip L, Guo K, Teng F, Dong J, Xu X, Leong DT, Sun X. Real-time magnetic resonance visualization of tumor acidosis as a precognition indicator of therapeutic efficacy. Bioact Mater. 2025 Jun 4;52:63-72. doi: 10.1016/j.bioactmat.2025.05.033 IF: 20.3 Q1 . PMID: 40530412 IF: 20.3 Q1 ; PMCID: PMC12172170 IF: 20.3 Q1 .
Others literatures
1. Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49 Suppl 2:24S-42S.
2. Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11(9):671-677.
3. Li W, Fan W, Bu W, et al. Smart manganese oxide nanoagents for magnetic resonance imaging-guided photothermal therapy. Chem Sci. 2017;8(3):1193-1200.
4. Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10(10):767-777.
5. Chen Z, Zhao P, Luo Z, et al. Glutathione-responsive disulfide bond-containing polymeric prodrug nanoplatforms for simultaneous diagnosis and therapy of tumors. Biomaterials. 2015;73:149-162.
6. DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2(5):e1600200.
7. Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta. 2008;1777(7-8):1028-1031.
8. Chen H, Zeng X, Ma Y, et al. Targeting tumor hypoxia and reactive oxygen species for effective photodynamic therapy. Chem Soc Rev. 2021;50(20):11933-11955.