Highlight
- A multifactorial diet significantly lowers plasma apolipoprotein C-III (ApoC-III) levels in individuals with type 2 diabetes compared to a MUFA-rich diet.
- Decreased ApoC-III correlates with reduced ectopic fat accumulation in liver and pancreas, key sites implicated in diabetes pathophysiology.
- Lower ApoC-III levels are associated with improved beta cell function, indicating better endogenous insulin secretion and glucose regulation.
Study Background and Disease Burden
Type 2 diabetes (T2D) is a major metabolic disorder characterized by insulin resistance and beta cell dysfunction, often accompanied by ectopic fat accumulation in organs such as the liver and pancreas. This ectopic fat is linked to impaired insulin secretion and worsened glycaemic control. Apolipoprotein C-III (ApoC-III) is a key modulator of triglyceride metabolism and increasingly recognized as a pathogenic factor in lipid abnormalities and metabolic disturbances in T2D. Elevated ApoC-III levels impair triglyceride clearance and are associated with increased risk of cardiovascular disease and insulin resistance.
Nutritional strategies targeting ectopic fat and related metabolic pathways have emerged as promising approaches to improve glycaemic outcomes and reduce complications. Prior studies have shown that diets rich in monounsaturated fatty acids (MUFA) can positively influence lipid metabolism. However, multifactorial diets incorporating polyunsaturated fats, dietary fiber, polyphenols, and vitamins may have superior effects on metabolic health including ectopic fat reduction.
The MEDEA randomized controlled trial was conducted to explore how such diets impact plasma ApoC-III levels and their associations with ectopic fat accumulation and beta cell function in individuals with T2D, addressing an unmet need to delineate diet-based modulation of ApoC-III and related metabolic outcomes.
Study Design
The MEDEA study was a randomized, controlled, parallel-group clinical trial enrolling 36 adults with clinically diagnosed type 2 diabetes, aged 35 to 75 years (20 men, 16 women). Participants were randomized to one of two isoenergetic dietary interventions for 8 weeks:
– MUFA-rich diet (n=16): Predominantly monounsaturated fats.
– Multifactorial diet (n=20): Rich in MUFA plus polyunsaturated fats, fiber, polyphenols, and vitamins.
Assessments included fasting and postprandial (3-hour test meal reflecting assigned diets) measurements of plasma glucose, insulin, and ApoC-III concentrations at baseline and after 8 weeks. Beta cell function was evaluated by calculating the ratio of insulin to glucose total area under the curve (AUC) from post-meal tests.
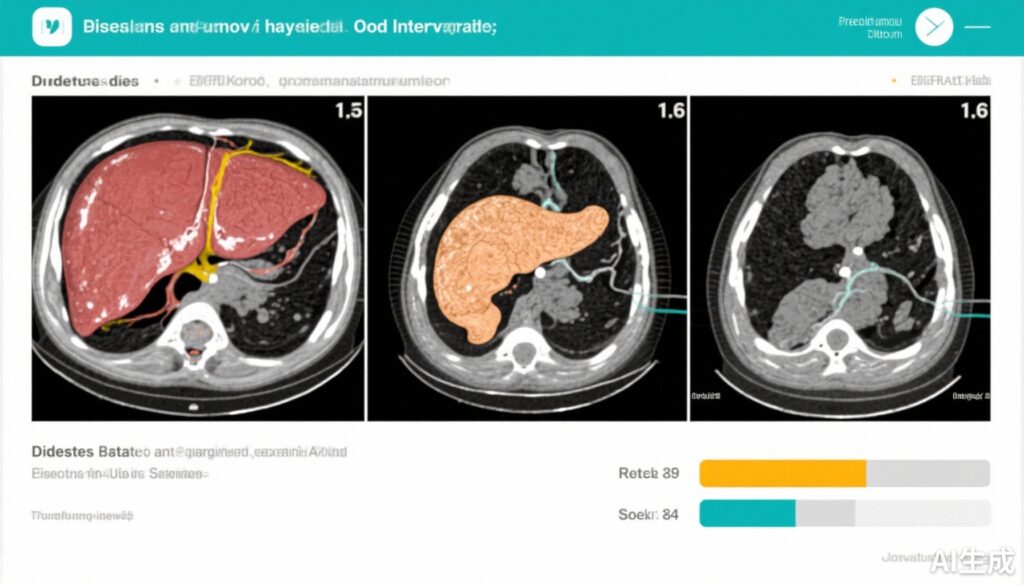
Ectopic fat quantification in the liver and pancreas was performed using advanced magnetic resonance imaging (MRI) techniques, enabling precise measurement of fat content changes over time.
Key Findings
The comparative analysis revealed that the multifactorial diet produced a significant reduction in postprandial ApoC-III levels compared to the MUFA-rich diet (difference in ApoC-III AUC change: -1.34 ± 6.01 vs +1.60 ± 5.56 g/l × 180 min; p=0.043). Although fasting ApoC-III reductions with the multifactorial diet showed a trend toward significance (-0.006 ± 0.040 vs +0.007 ± 0.048 g/l; p=0.070), the effect was more pronounced in the postprandial state, highlighting the diet’s impact on metabolic responses to meals.
Independent of diet assignment, reductions in fasting ApoC-III positively correlated with decreases in liver fat (r=0.357, p=0.032) and pancreatic fat (r=0.385, p=0.020). These findings underscore the close linked pathophysiology of ApoC-III levels and ectopic fat storage.
Furthermore, both fasting and postprandial decreases in ApoC-III were inversely correlated with improvements in beta cell function (r=-0.384, p=0.026 and r=-0.402, p=0.018 respectively). This suggests that lower ApoC-III levels may support enhanced endogenous insulin secretory capacity, potentially mediated by reduced lipotoxic stress on pancreatic islets.
Expert Commentary
Dr. Alessandra Annuzzi, co-investigator of the MEDEA study, highlights, “Our findings support the multifactorial dietary approach as a potent modulator of ApoC-III — a lipid regulator increasingly implicated in diabetes progression. The concomitant decrease in ectopic fat and improvement in beta cell function provides promising mechanistic insight linking diet, lipid metabolism, and glycaemic control.”
While the study offers pioneering insight, its exploratory nature and relatively small sample size represent limitations. Longer-term studies and larger cohorts are needed to validate these relationships and elucidate causality. Moreover, the multifactorial diet’s complexity makes isolating individual nutrient contributions challenging, warranting further mechanistic evaluation.
In the context of existing literature, these findings align with a growing body of evidence emphasizing the reduction of liver and pancreatic fat as a therapeutic target in T2D, and extend understanding by identifying ApoC-III modulation as a possible intermediary biomarker and therapeutic target.
Conclusion
The MEDEA randomized controlled study reveals that an isoenergetic multifactorial diet reduces plasma ApoC-III levels in individuals with type 2 diabetes, with these reductions closely associated with decreases in ectopic liver and pancreatic fat accumulation and improved beta cell function.
These results highlight the multifactorial diet’s potential as a dietary intervention to ameliorate lipid-related metabolic disturbances in T2D and underscore the clinical relevance of targeting ApoC-III-mediated pathways to improve beta cell health and metabolic outcomes.
Future clinical practice may incorporate ApoC-III level measurements as a metabolic marker for tailoring dietary therapies and monitoring ectopic fat changes. Further research is warranted to confirm these benefits and explore mechanistic underpinnings.
References
1. Costabile G, Salamone D, Della Pepa G, et al. ApoC-III and ectopic fat accumulation in individuals with type 2 diabetes: an exploratory analysis from the MEDEA randomised controlled study. Diabetologia. 2025 Sep;68(9):2036-2041. doi:10.1007/s00125-025-06464-w. Epub 2025 Jun 5. PMID: 40471240.
2. Goldberg IJ. Apolipoprotein C-III: Bridging hypertriglyceridemia and insulin resistance in diabetes. Curr Diab Rep. 2019;19(7):35.
3. Lim EL, et al. Reversal of type 2 diabetes: normalization of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54(10):2506-2514.
4. Petersen KF, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133-2223.