Highlights
- Nearly one-third (33%) of patients with relapsed/refractory multiple myeloma (RRMM) achieved ≥5 years of progression-free survival (PFS) after a single cilta-cel infusion.
- Long-term survivors showed specific biomarker profiles, including lower tumor burden and higher effector-to-target (E:T) cell ratios.
- Cilta-cel maintained a consistent safety profile, with no new long-term safety signals identified over five years of follow-up.
- This result challenges historical expectations for RRMM, where median PFS is typically less than six months, and median overall survival (OS) is about a year.
Clinical Background and Disease Burden
Multiple myeloma (MM) is a malignant, clonal proliferation of plasma cells within the bone marrow, leading to significant morbidity due to skeletal destruction, anemia, renal dysfunction, and immunosuppression. Despite advances in therapy, patients with relapsed/refractory MM (RRMM)—particularly those exposed to proteasome inhibitors, immunomodulatory drugs, and anti-CD38 monoclonal antibodies—have poor prognoses. Historically, median PFS for this population is less than six months, and median OS is approximately one year. The introduction of chimeric antigen receptor T-cell (CAR-T) therapies targeting B-cell maturation antigen (BCMA) has generated hope for improved depth and durability of response in RRMM.
Ciltacabtagene autoleucel (cilta-cel) is a second-generation BCMA-targeted CAR-T therapy. Early-phase trials, notably CARTITUDE-1, demonstrated high overall response rates (ORR) and deep remissions, but long-term durability and predictors of sustained benefit remained unclear.
Research Methodology
CARTITUDE-1 is an open-label, multicenter phase Ib/II study enrolling RRMM patients who had failed at least three prior therapies, including proteasome inhibitors, immunomodulators, and anti-CD38 antibodies. The trial consisted of a phase Ib portion (safety and dose-finding) and a phase II portion (efficacy, with ORR as the primary endpoint). Patients were subsequently entered into the longitudinal CARTINUE follow-up study, with annual assessments of disease status and survival for up to 15 years.
From July 2018 to October 2019, 97 patients received a single cilta-cel infusion. Disease progression was assessed per International Myeloma Working Group (IMWG) criteria. Key endpoints included OS and PFS (estimated by Kaplan-Meier method). Immunophenotypes were analyzed by flow cytometry, and serum biomarkers, including soluble BCMA (sBCMA), were measured. Effector-to-target (E:T) ratio was defined as the peak level (Cmax) of CAR-positive T cells divided by the pre-infusion sBCMA level. Safety was monitored continuously, including infections and neurological adverse events.
Key Findings
As of February 2025 (median follow-up: 61.3 months), 47 patients had died, 3 were lost to follow-up, and 2 discontinued due to progression. Forty-five (46.4%) remained alive under ongoing observation. The median OS for the entire cohort was 60.7 months—a remarkable outcome for this heavily pretreated population.
Notably, 32 patients (33%) experienced ≥5 years of PFS after a single cilta-cel infusion, requiring no further anti-myeloma therapy. Among these, 31 achieved a stringent complete response (sCR). Baseline features were broadly similar between long-term PFS achievers and those with earlier progression, though the former group had trends toward lower tumor burden (6.3% vs 17.4%), lower sBCMA (36.0 mg/L vs 58.5 mg/L, P=0.117), and lower marrow plasma cell infiltration (5.0% vs 24.0%, P=0.053).
Biomarker analyses revealed:
– Higher E:T ratio in ≥5-year PFS group (P=0.008).
– Greater CAR+ T cell Cmax (median 961/mL [IQR 482-1380] vs 450/mL [246-1040], P=0.028).
– Lower neutrophil-to-leukocyte ratios and higher T cell-to-neutrophil ratios at apheresis (P=0.03 and P=0.05, respectively).
– Higher proportion of naïve CAR+ T cells within the product (P=0.003).
– Higher baseline hemoglobin (P=0.001) and platelet counts (P=0.049).
– Numerically lower ferritin (P=0.158).
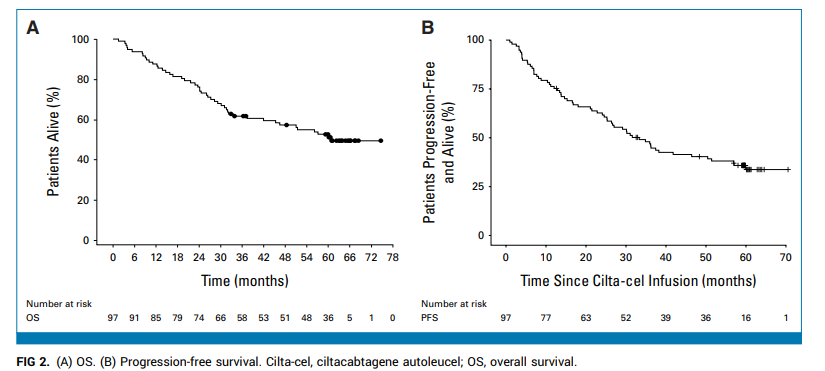
Regarding safety, in the ≥5-year PFS population, there were 2 cases of secondary primary malignancies (both solid tumors), 2 neurological adverse events, and 4 cases of grade ≥3 infection. Notably, there were no new reports of Parkinsonism or cranial nerve palsy.
Mechanistic Insights and Biological Plausibility
The association between long-term remission and higher E:T ratios, greater expansion of CAR+ T cells, and higher content of naïve T cells in the infused product suggests that both tumor burden and CAR-T cell quality at baseline are critical determinants of durable response. Lower sBCMA levels and less extensive marrow infiltration may reflect a more favorable microenvironment for CAR-T cell persistence and activity. Higher baseline hemoglobin and platelet counts further support the hypothesis that patients with less advanced disease and better marrow reserve are more likely to benefit from cilta-cel.
Expert Commentary
These findings are unprecedented in the RRMM setting and suggest the possibility of functional cure in a subset of patients. Current expert consensus underscores the transformative potential of BCMA-targeted CAR-T therapies, but also notes the need for improved patient selection and real-world validation. Guidelines may soon incorporate these biomarkers to refine eligibility and optimize outcomes.
Controversies and Limitations
Despite its strengths, this study is limited by its single-arm design, relatively small sample size, and lack of a randomized comparator. The cohort was heavily pretreated but may not reflect all RRMM populations. Biomarker findings, while hypothesis-generating, require validation in larger, independent cohorts. Long-term risks, including secondary malignancies, still warrant close surveillance.
Conclusion
The CARTITUDE-1 study demonstrates that a single infusion of cilta-cel can lead to ≥5 years of progression-free survival in one-third of highly refractory MM patients, without the need for maintenance therapy. This marks a paradigm shift in the management of RRMM, offering the hope of long-term disease control or even cure. Identification of predictive biomarkers such as tumor burden, E:T ratio, and product composition may further personalize CAR-T therapy. Ongoing research should focus on earlier intervention, combination strategies, and long-term safety monitoring.
References
1. Jagannath S, Martin TG, Lin Y, et al. Long-Term (≥5-Year) Remission and Survival After Treatment With Ciltacabtagene Autoleucel in CARTITUDE-1 Patients With Relapsed/Refractory Multiple Myeloma. J Clin Oncol. Published online June 3, 2025. doi:10.1200/JCO-25-00760 IF: 41.9 Q1 2. van de Donk NWCJ, Usmani SZ. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front Immunol. 2018;9:2134.
3. Munshi NC, Anderson KC, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705-716.