Highlight
- Lecanemab, a disease-modifying therapy for early-stage Alzheimer’s disease (AD), appears safe and effective when administered within a large, community-based health system, with manageable safety concerns.
- Rates of amyloid-related imaging abnormalities (ARIA), particularly among ApoE4 noncarriers, were lower than those observed in the pivotal CLARITY-AD clinical trial.
- Infusion reactions were more frequent than in clinical trials, but the majority were mild and rarely led to discontinuation.
- Ongoing safety surveillance and real-world data collection are critical to further refine patient selection and management strategies.
Background
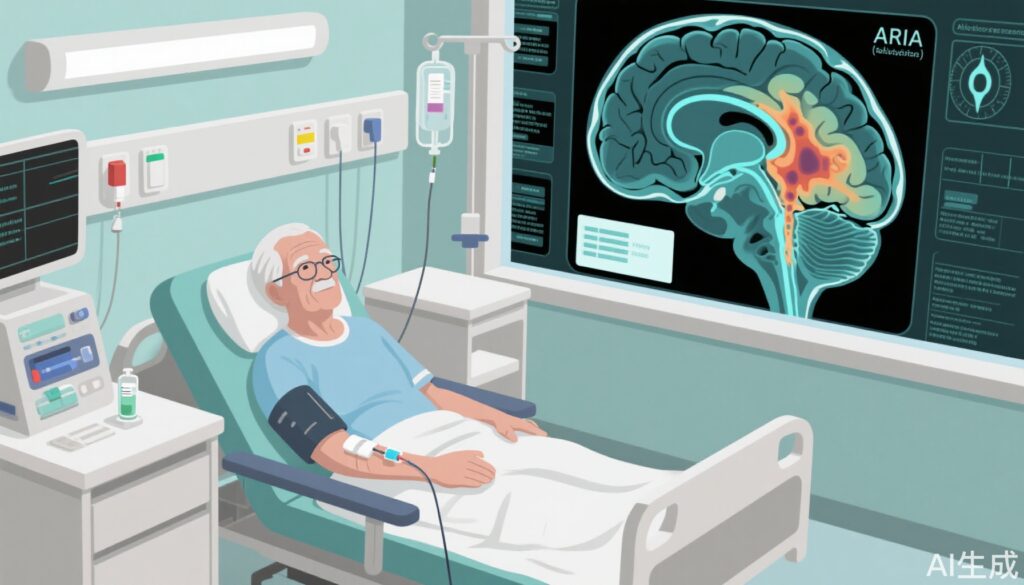
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the most common cause of dementia in older adults, affecting an estimated 6 million Americans. Until recently, standard care focused on symptom management rather than modifying disease progression. The approval of lecanemab, a monoclonal antibody targeting amyloid-beta (Aβ) aggregates, marked a new era as the first disease-modifying therapy shown to slow cognitive decline in early AD. However, its real-world safety—especially the risk of amyloid-related imaging abnormalities (ARIA), including vasogenic edema (ARIA-E) and microhemorrhages (ARIA-H)—remains a concern, particularly outside controlled clinical trial settings.
Study Overview and Methodological Design
To address the real-world safety and tolerability of lecanemab, Moughamian and colleagues conducted a retrospective, observational cohort study within the Sutter Health system, a large, community-based network in Northern California. The analysis included 210 patients (mean age 75 years, 52% women) treated with lecanemab between July 2023 and June 2024. All patients were screened for eligibility according to FDA labeling criteria for early-stage AD, including confirmation of amyloid pathology.
Participants were predominantly diagnosed with mild cognitive impairment (70%) or mild dementia (30%), reflecting the population targeted in the pivotal CLARITY-AD phase 3 trial. ApoE4 genotype was determined for all patients: 56% were heterozygous, 6% homozygous, and the remainder noncarriers. Safety monitoring included systematic assessment for infusion reactions, ARIA (via scheduled MRI), and adverse events, with endpoints mirroring those of CLARITY-AD for comparative interpretation.
Key Findings
Among the 210 patients:
- Infusion reactions occurred in 36% of patients (91% mild, 8% moderate, 1% severe), a higher rate than the 26% reported in the CLARITY-AD trial. Discontinuation due to reactions was noted in 14% of cases.
- ARIA was observed in 15.7% of patients (n=33), lower than the 21.5% (ARIA-E and ARIA-H combined) in CLARITY-AD.
- ARIA-H (microhemorrhage) occurred in 13.8%.
- ARIA-E (vasogenic edema) occurred in 7.6%.
- Severe radiographic ARIA was detected in 8 cases (3.8%), with only 3 symptomatic events.
- Rates of ARIA varied by ApoE4 status: 10.2% in noncarriers, 16.1% in heterozygotes, and 38.4% in homozygotes, mirroring genetic risk stratification seen in clinical trials.
- There were 5 deaths in the cohort, none attributed to lecanemab therapy.
When compared with CLARITY-AD:
- Infusion reactions: 36% (community) vs. 26% (CLARITY-AD lecanemab group).
- ARIA-E: 7.6% (community) vs. 13% (CLARITY-AD lecanemab group).
- ARIA-H: 13.8% (community) vs. 14% (CLARITY-AD lecanemab group).
- ARIA in ApoE4 noncarriers: 10.2% (community) vs. 13% (CLARITY-AD).
Mechanistic Insights and Pathophysiological Context
Lecanemab is a humanized IgG1 monoclonal antibody that selectively binds aggregated Aβ protofibrils, facilitating their clearance from the brain. This mechanism is believed to slow synaptic dysfunction and neurodegeneration in early AD. However, rapid removal of amyloid deposits, especially in ApoE4 carriers—who have impaired vascular amyloid clearance—increases the risk for ARIA. The observed lower ARIA rates among ApoE4 noncarriers in the Sutter Health cohort reinforce this genotype-dependent risk, supporting the need for individualized treatment decisions.
Clinical Implications
These real-world data suggest that lecanemab can be safely administered in community practice settings outside academic trial centers, provided that careful patient selection, ApoE4 genotyping, and MRI monitoring are rigorously applied. The majority of infusion reactions were mild and manageable, and ARIA rates—particularly in noncarriers—were lower than previously reported. This supports broader access for early-stage AD patients, especially those at lower genetic risk for ARIA.
For clinicians, these findings underscore the importance of:
- Pre-infusion patient counseling on potential risks (especially ARIA and infusion reactions).
- Routine ApoE4 genotyping as part of shared decision-making.
- Scheduled MRI surveillance during the first 6 months of therapy.
- Prompt recognition and management of ARIA or infusion reactions to minimize harm and optimize adherence.
Limitations and Controversies
Despite its strengths, this study has several limitations:
- Retrospective, observational design with potential for selection and reporting biases.
- Lack of a true control group limits causal inference regarding comparative efficacy and safety.
- Short follow-up (up to 12 months) precludes assessment of long-term adverse events or durability of benefit.
- Generalizability remains uncertain, as the cohort was limited to a single integrated health system in Northern California.
Controversies persist regarding the cost-effectiveness of lecanemab, the clinical meaningfulness of its cognitive benefit, and the optimal patient population for initiation. The burden of frequent infusions and MRI monitoring may also limit scalability in less resourced settings.
Expert Commentary or Guideline Positioning
Dr. Armen J. Moughamian, cognitive neurologist and medical director at Sutter Health, commented: “We conclude that lecanemab can be safely administered in a large, community-based health care system and that ARIA is a manageable side effect when appropriate patients are selected for treatment.”
Current consensus guidelines from the Alzheimer’s Association and American Academy of Neurology recommend that disease-modifying therapies be reserved for patients with early symptomatic AD, confirmed amyloid pathology, and close radiographic monitoring, particularly in ApoE4 carriers.
Conclusion
Real-world experience from Sutter Health demonstrates that lecanemab is feasible and generally safe for early-stage Alzheimer’s disease in community practice, with ARIA rates lower than those seen in clinical trials, especially for ApoE4 noncarriers. Most infusion reactions are mild and manageable. Continued post-marketing surveillance and data collection are essential to further refine risk stratification and optimize outcomes.
References
- van Dyck CH, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948 IF: 78.5 Q1
- Leqembi (lecanemab-irmb) [prescribing information]. Eisai Inc.; 2024.
- Alzheimer’s Association. Disease-Modifying Therapies in Alzheimer’s Disease: Position Statement. 2024.
- https://www.leqembihcp.com/about-leqembi/study-2-clarity-ad. Accessed July 25, 2025.
- Ashmawy RE, Okesanya OJ, Ukoaka BM, Daniel FM, Ezedigwe SG, Agboola AO, Ahmed MM, Ogaya JB, Amisu BO, Adigun OA, Oluwakemi OG, Hamza AM, Mourid MR, Kouwenhoven M, Lucero-Prisno DE 3rd. Exploring the efficacy and safety of lecanemab in the management of early Alzheimer’s disease: A systematic review of clinical evidence. J Alzheimers Dis. 2025 Jun;105(3):714-728. doi: 10.1177/13872877251331640.