Highlight
1. Single-cell multiomics reveals sequential immune cell alterations underpinning HBV-ACLF progression, identifying early inflammatory VCAN+CD14+ monocytes and later CXCR2+ neutrophil-driven immunosuppression.
2. Dysfunction and exhaustion of cytotoxic T-cells correlate with disease worsening and are linked to CXCR2+ neutrophil activity.
3. Pharmacological CXCR2 inhibition in animal models mitigates neutrophil infiltration, restores T-cell function, and improves clinical outcomes.
4. Identification of six immune cellular modules provides a framework for patient stratification and early therapeutic intervention windows.
Study Background and Disease Burden
Acute-on-chronic liver failure (ACLF) represents a severe clinical syndrome characterized by rapid deterioration of liver function in patients with chronic liver disease, often precipitated by infections or reactivation of hepatitis B virus (HBV). This syndrome is associated with high short-term mortality due to systemic inflammation, multi-organ failure, and immune dysregulation. HBV-related ACLF (HBV-ACLF) is particularly prevalent in regions endemic for HBV infection and remains a critical challenge owing to its complex immunopathogenesis and limited therapeutic options. Immune cell dysfunction, notably involving monocytes, neutrophils, and T-cells, has been implicated in ACLF, yet the dynamic trajectory of these immune alterations during the disease course remains incompletely understood. Detailed characterization of immune responses at single-cell resolution offers the potential to uncover critical cellular players and pathways driving disease progression, enabling precision therapeutic strategies.
Study Design
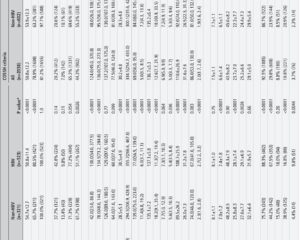
This longitudinal study employed single-cell RNA sequencing and single-cell proteomics to analyze peripheral blood mononuclear cells (PBMCs) sampled from 17 hospitalized patients with HBV-ACLF, representing progressive (n=6), stable (n=5), and recovering (n=6) disease phases. Controls consisted of 15 subjects: 5 with liver cirrhosis, 5 with chronic hepatitis B, and 5 healthy volunteers. A total of 45 PBMC samples underwent detailed transcriptional and proteomic profiling to investigate the immune landscape across disease stages. Functional and mechanistic validations were conducted in vivo using ACLF mice models and in vitro cell culture systems to dissect the causal roles of identified immune subsets and signaling pathways.
Key Findings
Immune Cell Dynamics and HBV-ACLF Progression
Early in ACLF (ACLF-1), VCAN+CD14+ monocytes expressing elevated interferon-stimulated genes (ISGs) and inflammatory mediators expand markedly. This subset is likely driven by HBV viral relapse, fueling the initial systemic inflammatory storm observed clinically. These monocytes demonstrate increased chemotaxis, antigen presentation, and proinflammatory cytokine production, contributing to immune activation.
Subsequently, apoptotic hepatocytes promote enrichment of hyperinflammatory CXCR2+ neutrophils and CD163+ monocytes, especially in patients with progressive ACLF. These cells correlate with worsening clinical status and serve as biomarkers of disease deterioration. CXCR2+ neutrophils not only drive inflammation but also exhibit immunosuppressive properties, inducing exhaustion and functional impairment of cytotoxic CD8+ T-cells, which are significantly depleted in progressive patients.
Immunosuppressive Mechanisms and T-cell Exhaustion
Cytotoxic T-cells show progressive functional decline during ACLF, marked by expression of exhaustion-related molecules and reduced effector cytokine production. CXCR2+ neutrophils mediate this suppression through direct and indirect mechanisms, establishing an immunosuppressive microenvironment that impedes effective antiviral immunity and tissue repair.
Therapeutic Targeting of CXCR2
Pharmacological inhibition of CXCR2 in ACLF mouse models results in significant reduction of neutrophil infiltration into inflamed liver tissue, restoration of cytotoxic T-cell function, and improvement in survival and liver function parameters. This experimental treatment highlights CXCR2 as a promising therapeutic target to interrupt the deleterious neutrophil-T-cell axis during ACLF progression.
Immune Cellular Modules and Patient Stratification
The study identifies six distinct immune cellular modules (CMs) through unbiased single-cell clustering and module analysis. CM2 and CM6 exhibit strong predictive value for adverse clinical outcomes, offering potential as prognostic biomarkers. CM3 appears enriched during the early ACLF phase, suggesting an optimal time window for therapeutic intervention before irreversible organ damage ensues.
Expert Commentary
The comprehensive single-cell multiomics approach elegantly maps the temporal and functional heterogeneity of peripheral immune cells in HBV-ACLF, enhancing our mechanistic understanding of this complex syndrome. The identification of sequential immune events—from the early VCAN+CD14+ monocyte-driven inflammatory surge to the later CXCR2+ neutrophil-induced immunosuppressive environment—provides a nuanced framework for disease staging and targeted therapy development.
Notably, the demonstration that CXCR2 pharmacological blockade reverses detrimental immune cell interactions and improves outcomes in murine models is translationally significant, advocating for clinical trials of CXCR2 inhibitors in HBV-ACLF patients. This study also underscores the importance of cytotoxic T-cell preservation and reactivation as a therapeutic goal to restore antiviral immunity and prevent disease progression.
Limitations include the relatively small patient cohort and peripheral blood sampling, which may not fully recapitulate intrahepatic immune dynamics. Future studies integrating liver tissue single-cell analyses and expanded cohorts could validate and extend these findings. Additionally, the heterogeneity of HBV-ACLF etiologies and co-morbidities warrants personalized immunophenotyping to optimize patient-specific interventions.
Conclusion
This longitudinal single-cell multimodal investigation delineates the dynamic immunopathogenic trajectory of HBV-ACLF, revealing critical immune cell subsets and mechanisms driving disease evolution. The study provides a compelling rationale for precision immunomodulatory therapies targeting CXCR2+ neutrophils and aims to preserve cytotoxic T-cell function. Integrating immune cellular modules into clinical practice could enhance patient stratification and identify early intervention windows, ultimately improving outcomes in this life-threatening disease. Continued translational efforts will be pivotal to advancing novel therapies and refining clinical management in HBV-ACLF.
References
Liang X, Luo J, Zhou Q, et al. Single-cell multimodal analysis reveals the dynamic immunopathogenesis of HBV-ACLF progression. Gut. 2025 Aug 21:gutjnl-2024-333308. doi: 10.1136/gutjnl-2024-333308. Epub ahead of print. PMID: 40841166.
Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013 Mar;144(7):1426-1437.e9. doi: 10.1053/j.gastro.2013.02.042.
Schneeberger PHH, Bansal S, Hamann A. Cytotoxic T lymphocyte exhaustion in chronic infection and cancer: an overview. Immunology. 2021;164(2):129-134. doi:10.1111/imm.13345.
Morita R, Burkett PR, Godec J, et al. CXCR2 regulates neutrophil recruitment and tissue injury in liver inflammation. J Immunol. 2012;189(8):4675-4685. doi:10.4049/jimmunol.1201092.