Introduction
Heart failure with preserved ejection fraction (HFpEF) is the most prevalent form of heart failure, frequently complicating cardiometabolic conditions such as obesity and type 2 diabetes. These comorbidities exacerbate symptom burden, functional impairment, and cardiovascular risk. Despite this substantial disease burden, effective treatments for HFpEF, particularly in populations with metabolic dysfunction, remain limited.
Recent randomized controlled trials have demonstrated that semaglutide—a glucagon-like peptide-1 (GLP-1) receptor agonist—and tirzepatide—a dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonist—improve clinical symptoms in obesity-related HFpEF. However, these studies were limited by small sample sizes, few clinical events, and restrictive eligibility criteria, limiting generalizability and impeding regulatory approval and clinical guideline incorporation.
This study aimed to generate robust real-world evidence on the effectiveness and safety of semaglutide and tirzepatide in patients with cardiometabolic HFpEF using large, nationally representative US claims databases. The study benchmarked analytic approaches against pivotal trials (STEP-HFpEF DM for semaglutide and SUMMIT for tirzepatide), expanded eligibility to include broader patient populations, and conducted a head-to-head comparison between semaglutide and tirzepatide to support clinical decision-making.
Study Design and Methods
Using data from Medicare Parts A, B, and D, Optum Clinformatics Data Mart, and Merative MarketScan (2018–2024), the investigators constructed five active-comparator new-user cohort studies. These included cohort emulations of the STEP-HFpEF DM (semaglutide) and SUMMIT (tirzepatide) trials to benchmark study design, before expanding eligibility criteria to encompass patients reflective of routine clinical practice.
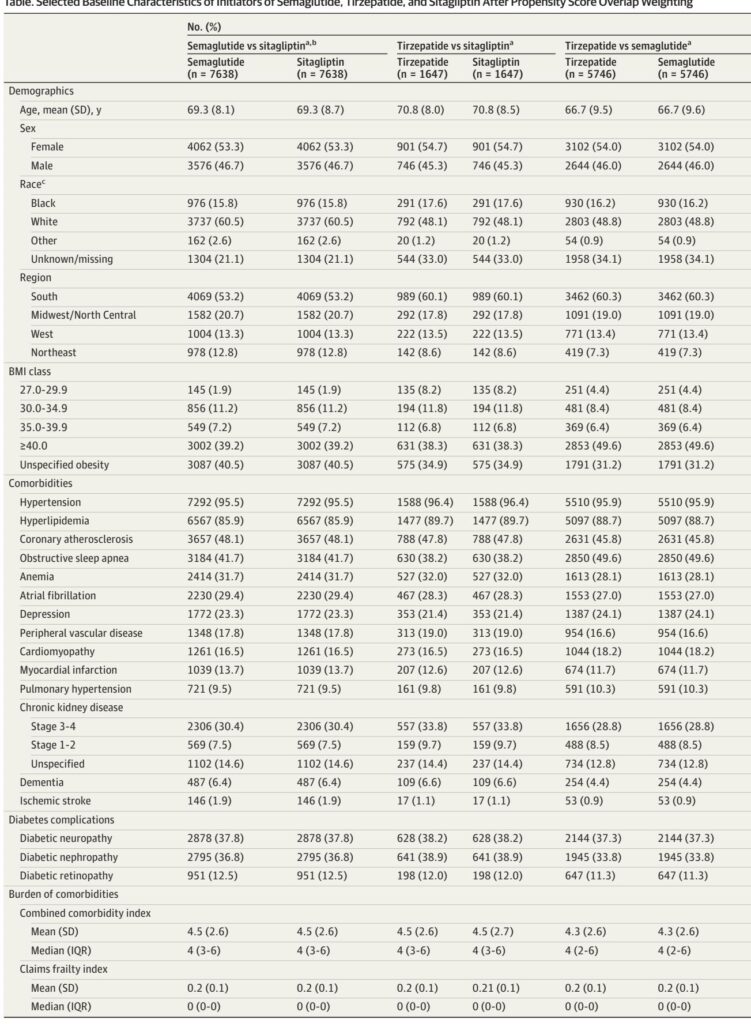
New users of semaglutide or tirzepatide were compared against new users of sitagliptin, a DPP-4 inhibitor considered a placebo proxy for heart failure outcomes due to neutral effects on heart failure in prior trials. A final cohort directly compared new users of tirzepatide vs semaglutide. Inclusion criteria required documented diagnosis of HFpEF, obesity (with BMI cutoffs aligned or expanded from trials), type 2 diabetes, and a stable clinical profile in terms of cardiovascular and metabolic comorbidities.
The primary outcome was a composite of hospitalization for heart failure or all-cause mortality within 52 weeks following treatment initiation. Secondary outcomes included broader composites of urgent heart failure visits requiring intravenous diuretics and individual components of the primary composite. Safety endpoints evaluated gastrointestinal adverse events, serious bacterial infections, and urinary tract infections.
Confounding was rigorously addressed through propensity score overlap weighting incorporating a comprehensive range of covariates, including demographics, cardiometabolic burden, comorbidities, concomitant medication, health care utilization, frailty indices, and laboratory measures where available.
Benchmarking metrics evaluated concordance between database emulations and randomized trial results, enhancing confidence in methodological validity. Negative control outcomes (lumbar radiculopathy and abdominal hernia) were included to assess potential residual confounding. Prespecified subgroup analyses examined treatment effects by age, sex, and BMI.
Key Findings
1. Patient Populations: After applying inclusion and exclusion criteria, cohorts consisted of 58,333 patients for semaglutide vs sitagliptin, 11,257 for tirzepatide vs sitagliptin, and 28,100 for tirzepatide vs semaglutide comparisons under expanded eligibility. Baseline characteristics were well-balanced following propensity score weighting, with mean ages ranging 66.7–70.8 years, female representation approximately 53–55%, and mean BMI between 36.6 and 40.2.
2. Benchmarking: Database emulations of STEP-HFpEF DM and SUMMIT trials demonstrated high agreement with clinical trial hazard ratios for heart failure hospitalization and mortality outcomes, validating the analytic approach.
3. Primary Outcome – Semaglutide vs Sitagliptin: The 1-year risk of hospitalization for heart failure or all-cause mortality was 5.5% (95% CI, 4.9–6.1) in the semaglutide group versus 8.6% (95% CI, 7.9–9.3) in the sitagliptin group, corresponding to a 42% relative risk reduction (HR 0.58; 95% CI, 0.51–0.65). The number needed to treat (NNT) to prevent one event was 31.
4. Primary Outcome – Tirzepatide vs Sitagliptin: Tirzepatide was associated with a 58% relative risk reduction (HR 0.42; 95% CI, 0.31–0.57), with 1-year event rates of 3.6% vs 7.5%, and NNT of 26.
5. Head-to-Head Comparison – Tirzepatide vs Semaglutide: No meaningful difference in the primary composite outcome was observed (HR 0.86; 95% CI, 0.70–1.06), with near-identical 1-year observed risks (3.3% vs 3.4%), suggesting comparable effectiveness.
6. Secondary Outcomes: Both semaglutide and tirzepatide reduced broader composite outcomes and individual endpoints including heart failure hospitalization and all-cause mortality compared with sitagliptin, with consistent effect sizes.
7. Safety: No substantial increases were observed in gastrointestinal adverse events, serious bacterial infections, or urinary tract infections with semaglutide or tirzepatide compared with sitagliptin or when compared head-to-head.
8. Subgroup and Sensitivity Analyses: Treatment effects were consistent across age, sex, and BMI subgroups, and robust across various sensitivity analyses, including use of broader outcome definitions and intention-to-treat approaches. Negative control analyses showed no significant associations, supporting validity.
Expert Commentary
These real-world findings reinforce and extend early clinical trial evidence supporting the use of GLP-1 receptor agonist–based therapies in cardiometabolic HFpEF. The rapid onset of cardiovascular benefits observed aligns with the SELECT trial results, suggesting early cardiometabolic mechanisms beyond weight loss may mediate improved heart failure outcomes.
While tirzepatide has demonstrated superior glycemic and weight-loss effects in other settings, this study found no clear advantage over semaglutide for heart failure reduction, supporting clinicians’ use of either agent based on patient-specific factors, availability, and tolerability.
Importantly, the study exemplifies the strength of rigorous observational research, leveraging large databases to confirm and expand upon randomized trial findings, thus informing regulatory and guideline decisions, and addressing gaps in populations underrepresented in clinical trials.
Limitations include the observational design, potential residual confounding despite extensive adjustment, reliance on prescription dispensing data for treatment exposure, and lack of standardized clinical measures such as heart failure symptom assessment or ejection fraction measurements. However, benchmarking against randomized trials and the use of validated claims algorithms mitigate some concerns.
Conclusion
In patients with obesity-related HFpEF and type 2 diabetes, initiation of semaglutide or tirzepatide is associated with substantial reductions (>40%) in hospitalization for heart failure or all-cause mortality compared with sitagliptin, a neutral glucose-lowering comparator. No meaningful difference in effectiveness between tirzepatide and semaglutide was detected.
These findings support the integration of GLP-1 receptor agonist therapies into the management of cardiometabolic HFpEF and underscore the value of real-world evidence in complementing randomized trials to guide clinical and regulatory decisions.
Reference
Krüger N, Schneeweiss S, Fuse K, Matseyko S, Sreedhara SK, Hahn G, Schunkert H, Wang SV. Semaglutide and Tirzepatide in Patients With Heart Failure With Preserved Ejection Fraction. JAMA. 2025 Aug 31. doi: 10.1001/jama.2025.14092. Epub ahead of print. PMID: 40886075.