Study Background and Disease Burden
Metabolic dysfunction-associated steatohepatitis (MASH), also known as non-alcoholic steatohepatitis (NASH), represents an escalating global health concern characterized by hepatic steatosis, inflammation, hepatocellular injury, and fibrosis. It is strongly linked to metabolic syndrome, obesity, and type 2 diabetes mellitus, contributing significantly to liver-related morbidity and mortality. Despite the substantial burden of MASH, effective pharmacologic therapies remain limited, underscoring an unmet medical need for novel, mechanism-targeted treatments.
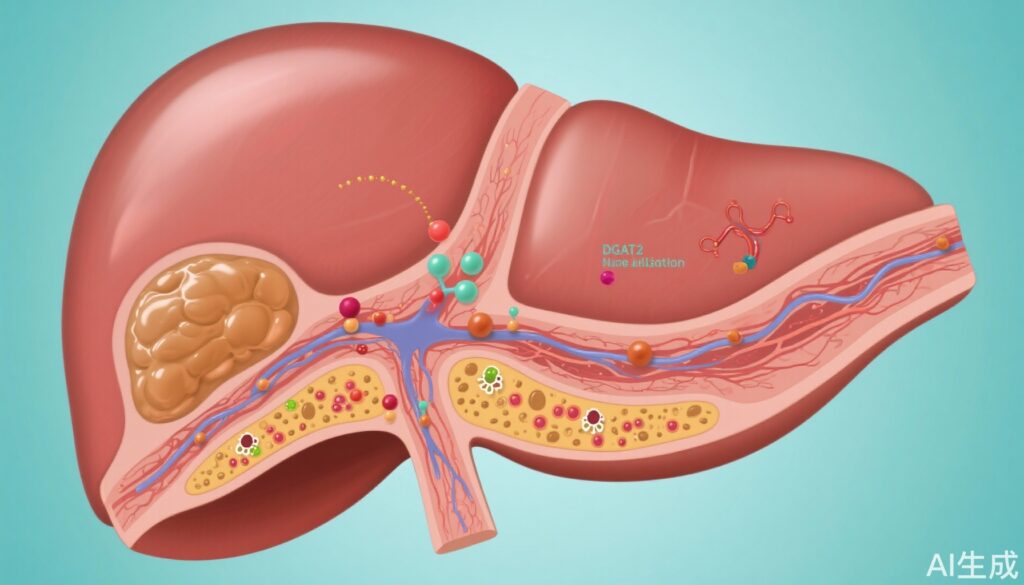
De novo lipogenesis is a critical metabolic pathway implicated in the pathogenesis of MASH, promoting lipotoxicity that exacerbates inflammation, hepatocyte damage, and progression to fibrosis. Diacylglycerol O-acyltransferase 2 (DGAT2), a central enzyme in triglyceride synthesis, is a compelling target to attenuate hepatic fat accumulation and its downstream pathological consequences. ION224 is a liver-directed antisense oligonucleotide designed to inhibit DGAT2 messenger RNA, thereby reducing DGAT2 protein expression and activity. Preclinical studies have suggested potent suppression of lipogenesis and improvement of hepatic histology with this therapeutic strategy.
Study Design
ION224-CS2 was a robust adaptive, two-part, multicentre, randomised, double-blind, placebo-controlled phase 2 trial conducted across 43 clinical sites in the USA and Puerto Rico. The study enrolled adults aged 18 to 75 years who had biopsy-confirmed MASH with fibrosis stages F1 to F3 and baseline liver steatosis ≥10%, thereby representing a clinically relevant population with significant disease.
Part 1 of the trial randomly assigned participants in a 1:1:1:1 ratio to receive subcutaneous injections once monthly of ION224 at doses of 60 mg, 90 mg, or 120 mg, or placebo. Following an interim analysis focusing on safety and liver steatosis, the second part employed a 2:1 randomisation to ION224 90 mg or 120 mg versus placebo. The primary efficacy endpoint was achievement at week 51 of a ≥2-point reduction in the Non-Alcoholic Fatty Liver Disease Activity Score (NAS), coupled with a ≥1-point improvement in hepatocellular ballooning or lobular inflammation, without worsening of fibrosis. The primary analysis was performed on a predefined per-protocol set, including participants who adhered to the dosing schedule (minimum 10 of 13 doses, no three consecutive missed doses) and completed the endpoint liver biopsy.
Key Findings
Between June 2021 and December 2022, 160 participants were randomised: 23 to ION224 60 mg, 45 to 90 mg, 46 to 120 mg, and 46 to placebo. Among them, 123 participants fulfilled the per-protocol criteria for the primary analysis.
The primary endpoint was met in 46% (18/39) of participants treated with 90 mg ION224 and 59% (20/34) with 120 mg, compared with 19% (6/32) in the placebo group. The risk difference versus placebo was 27.4% (95% CI 6.7–48.1, p=0.0094) for 90 mg and 40.1% (18.7–61.4, p=0.0002) for 120 mg dosing, demonstrating statistically significant and clinically meaningful histological improvements.
Notably, these improvements in NAS and hepatocellular ballooning or lobular inflammation occurred without worsening of fibrosis, an important consideration for long-term outcomes. The study also found that histological benefit was independent of bodyweight changes, suggesting that ION224’s efficacy is not contingent upon weight loss.
Safety analysis showed that ION224 was well tolerated. Although adverse events occurred frequently (94% in ION224 groups versus 89% in placebo), they were mainly mild to moderate. Importantly, no deaths or treatment-related serious adverse events were reported, and the safety profile was consistent across dosages.
Expert Commentary
The ION224-CS2 trial offers pioneering clinical evidence supporting antisense oligonucleotide-mediated DGAT2 inhibition as a viable therapeutic avenue in MASH management. The adaptive trial design and rigorous per-protocol analysis enhance the reliability of findings. The histological endpoint—reduction in NAS with preservation of fibrosis—aligns well with regulatory expectations and could translate into reduced progression to cirrhosis.
The independence from weight loss phenomena is particularly relevant clinically, as many patients with MASH struggle with sustained weight management. This characteristic raises the potential for combination therapy, for example, with GLP-1 receptor agonists that promote weight reduction and improve metabolic parameters.
Limitations include the relatively small per-protocol population and the 51-week duration, which, although sufficient for phase 2 evaluation, warrants longer-term studies to assess durability and impact on fibrosis regression and clinical outcomes. Additionally, while the safety profile is encouraging, monitoring in larger and more diverse populations will be important to confirm these results.
Conclusion
The ION224-CS2 phase 2 trial marks a significant advance in MASH therapeutics by demonstrating that liver-directed antisense inhibition of DGAT2 with ION224 is both efficacious and safe over 51 weeks. The observed histological improvements in liver inflammation and hepatocellular injury, without fibrosis worsening or dependency on weight loss, highlight ION224 as a compelling candidate for further clinical development. Future studies should focus on long-term outcomes, fibrosis regression, and the integration of ION224 into combination regimens with other metabolic interventions to maximize patient benefit.
References
Loomba R, Morgan E, Yousefi K, Li D, Geary R, Bhanot S, Alkhouri N; ION224-CS2 Investigators. Antisense oligonucleotide DGAT-2 inhibitor, ION224, for metabolic dysfunction-associated steatohepatitis (ION224-CS2): results of a 51-week, multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2025 Aug 23;406(10505):821-831. doi: 10.1016/S0140-6736(25)00979-1. PMID: 40849139.