Highlight
– Multiple prospective human studies report detectable microplastics and nanoplastics (MNPs) in coronary blood, cardiopulmonary-bypass (CPB)–exposed blood, and carotid atheromas.
– MNP presence correlates with local and systemic inflammatory markers and with higher risk of major adverse cardiovascular events (MACE) in observational cohorts.
– Findings are biologically plausible given preclinical evidence but are subject to potential contamination, methodological heterogeneity, and residual confounding; causality remains unproven.
Study background and disease burden
Microplastics (particles <5 mm) and nanoplastics (<1000 nm) are pervasive environmental contaminants produced when larger plastic items degrade by photochemical, mechanical, or biological processes. Global exposure occurs via ingestion, inhalation, and dermal contact; studies have detected MNPs in human biological materials including placenta, breast milk, urine, blood, lungs, and liver. Emerging toxicologic literature—cell culture and animal models—shows MNPs can trigger oxidative stress, endothelial inflammation, apoptosis, altered heart rate, myocardial dysfunction and fibrosis, and vascular dysfunction. Cardiovascular disease (CVD) remains the leading cause of death worldwide. If MNP exposure contributes to CVD risk, the public-health impact could be substantial. Until recently, however, direct human data linking MNP burden to vascular pathology and clinical events were sparse.
Study design and methods (summary of the three studies)
Three prospective observational human studies reporting MNP detection and clinical associations are summarized here.
– Study 1: Coronary blood in myocardial infarction (Zhang et al., J Hazard Mater 2025). Prospective observational cohort of 142 patients undergoing coronary angiography for myocardial infarction; coronary blood samples analyzed by pyrolysis–gas chromatography–mass spectrometry (Py‑GC/MS) for polymer types and concentrations. Follow-up for clinical events averaged 31.5 months for 110 patients.
– Study 2: CPB exposure in children (Wu et al., J Hazard Mater 2025). Prospective observational study in 22 children undergoing congenital heart surgery with cardiopulmonary bypass. Paired blood samples pre‑ and post‑CPB were analyzed by Py‑GC/MS and laser direct infrared spectroscopy (LDIR), with scanning electron microscopy for particle visualisation. Clinical laboratory correlations (white blood cell and neutrophil counts) were examined.
– Study 3: Atheromas and cardiovascular events (Marfella et al., N Engl J Med 2024). Multicenter prospective observational study of 304 patients undergoing carotid endarterectomy for asymptomatic carotid stenosis. Excised plaques were analyzed using Py‑GC/MS, stable‑isotope analysis, and electron microscopy; inflammatory biomarkers were assessed by ELISA and immunohistochemistry. Primary end point: composite of myocardial infarction, stroke, or death during follow-up (mean ~34 months).
Common laboratory modalities include Py‑GC/MS for polymer identification/quantification and microscopy/spectroscopy for particle visualization and elemental analysis. These methods provide complementary chemical and morphological information but have different strengths and limitations.
Key findings
This section summarizes principal results from each study and contrasts their implications.
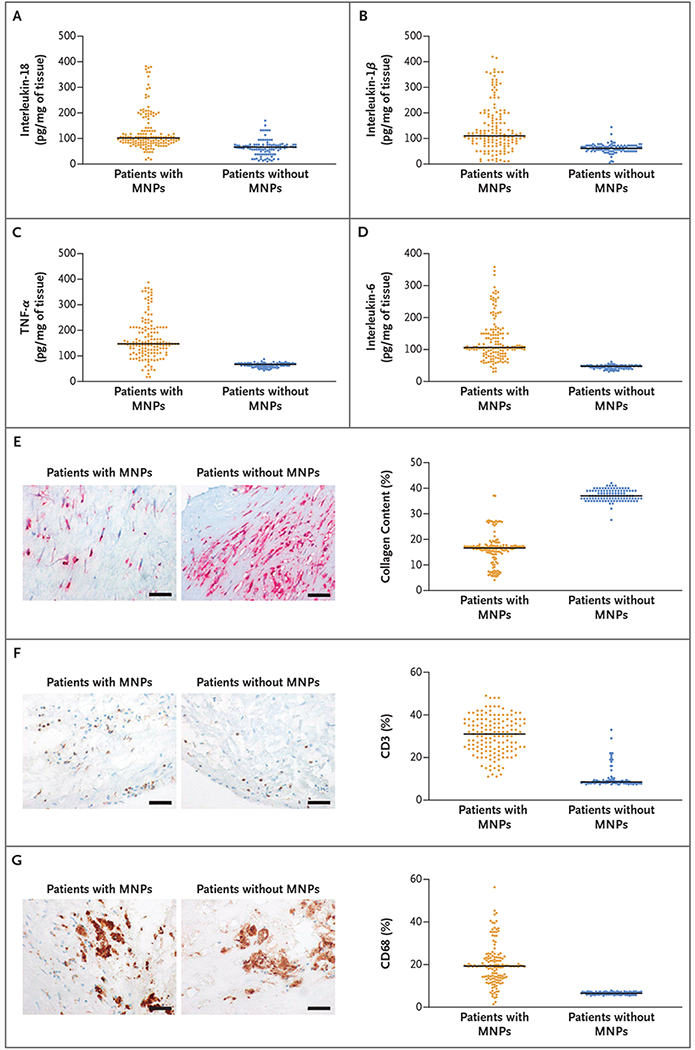
– Coronary blood in MI (Zhang et al.): Of 110 patients completing follow-up, polymer detection in coronary blood was common: polystyrene in 43.6%, polyethylene in 71.8%, polyvinyl chloride (PVC) in 95.4%, and polyamide 66 in 61.8%. Higher coronary PVC concentrations were observed in patients who experienced major adverse cardiac events (MACE). PVC levels correlated with proinflammatory cytokines (IL‑1β, IL‑6, IL‑18, and TNF‑α). Regression analysis reported an adjusted odds ratio for MACE associated with PVC of 1.090 (95% CI 1.032–1.1523, P = 0.002) per unit (study units not fully standardized in the manuscript); notably, the authors state that each 10-unit increase in PVC was associated with a 2.374-fold higher risk of MACE (OR 2.374; 95% CI 1.366–4.128; P = 0.002). Additional analysis of blood and thrombus samples in 21 MI patients found PVC levels in coronary thrombi correlated with inflammatory markers and monocyte/macrophage infiltration.
– CPB in children (Wu et al.): Total MNP concentration in blood rose significantly after CPB (p < 0.0001). Polymer‑specific increases were statistically significant for polystyrene (p = 0.046), polyethylene (p = 0.038), polypropylene (p < 0.0001), PVC (p < 0.0001), and polyamide 6 (p = 0.027). CPB duration correlated with MNP exposure (r = 0.43, P = 0.047). Increases in MNP exposure correlated with increases in white blood cell count (r = 0.52, P = 0.013) and neutrophils (r = 0.46, P = 0.029). LDIR confirmed a higher post‑CPB particle count (p = 0.015). The authors conclude that CPB circuits—constructed largely of plastic—can be a direct source of acute blood exposure to MNPs.
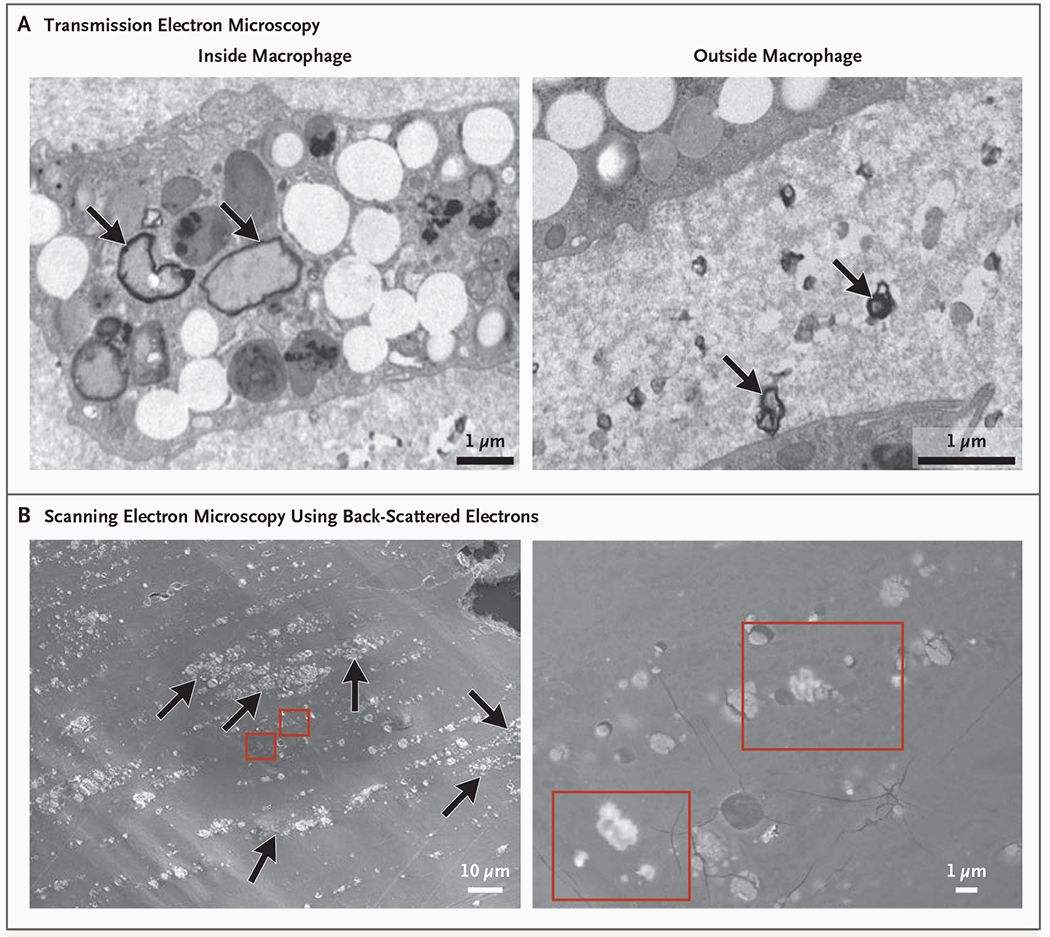
– Atheroma and cardiovascular events (Marfella et al.): Polyethylene was detected in 150 of 257 evaluable plaques (58.4%), with a mean level reported as 21.7 ± 24.5 μg per milligram of plaque; PVC was detected in 31 patients (12.1%), mean 5.2 ± 2.4 μg/mg. Electron microscopy revealed jagged particles embedded among plaque macrophages; radiographic elemental analysis indicated chlorine in some particles (consistent with PVC). Patients whose plaques contained MNPs had higher incidence of the composite primary end point (hazard ratio 4.53; 95% CI 2.00–10.27; P < 0.001) compared with patients whose plaques did not show MNPs.
Electron Microscopy Analysis of Atheromatous Plaque.
Inflammatory Markers in Plaque Samples.
Comparative interpretation: Across studies, MNPs were frequently detected and associated with markers of inflammation and with adverse clinical outcomes (in the carotid plaque and MI cohorts). The CPB study demonstrates a plausible iatrogenic pathway for acute intravascular MNP exposure and an observable inflammatory response in the short term.
Expert commentary and critical appraisal
Biologic plausibility: The association between MNPs and vascular inflammation is consistent with in vitro and animal data showing particle‑induced oxidative stress, endothelial activation, cytokine release, and immune cell recruitment. MNPs may also carry adsorbed chemical additives (plasticizers, flame retardants) or microbial components that amplify inflammatory responses.
Methodologic strengths: All three studies are prospective and employ modern analytic techniques (Py‑GC/MS, LDIR, electron microscopy) providing chemical specificity and, in some cases, visualized particle localization within tissue. The carotid study’s multicenter design and clinical end point strengthen external validity for symptomatic events.
Key limitations and alternative explanations:
– Contamination and analytic standardization: Plastics are ubiquitous; rigorous blank and procedural controls are essential. Published studies vary in contamination control reporting and in how polymer concentration units are presented, complicating between‑study comparisons.
– Measurement and particle characterization: Py‑GC/MS provides polymer mass and chemical signature but not particle size or morphology; microscopy can visualize particles but is limited in quantitative throughput and chemical specificity. Combining techniques is an advance but standard protocols are not yet universally adopted.
– Observational design and confounding: Associations do not prove causation. In the carotid study, patients with plaques containing MNPs could differ in unmeasured ways (geographic, occupational exposures, diet, socioeconomic factors) that influence cardiovascular risk. Residual confounding by established CVD risk factors remains possible despite adjustment.
– Small sample sizes for some analyses: The CPB pediatric cohort (n=22) and the thrombus subset (n=21) are small; findings should be considered exploratory.
– Unit interpretation and dose–response: The MI study reports an intriguing dose association for PVC and MACE, but interpretation requires clarity on measurement units and potential nonlinearity.
Clinical implications: The evidence is provocative and moves the needle from theoretical risk toward measurable human exposure linked to inflammation and to adverse outcomes in observational cohorts. However, it falls short of demonstrating causality or establishing exposure thresholds for clinical decision‑making. Current data do support two practical considerations: (1) the possibility of acute iatrogenic exposure from plastic medical devices (e.g., CPB circuits) and (2) the need for systematic contamination controls in research and for regulatory attention to device biocompatibility and particulate shedding.
Conclusion and clinical takeaways
Recent human studies document MNPs in coronary blood, CPB‑exposed blood, and carotid plaques and report associations with inflammation and worse cardiovascular outcomes. These data provide plausible human corroboration of preclinical toxicology and suggest MNPs could act as an overlooked environmental or iatrogenic risk factor in CVD. However, important caveats include potential contamination, heterogeneous analytic methods, residual confounding, and the observational nature of the evidence. Clinicians should be aware of these emergent data, support further high‑quality research, and engage with device manufacturers and regulators on minimizing plastic particle shedding in invasive procedures.
Research agenda and policy priorities
– Standardize sampling, blank controls, and analytic pipelines (harmonized Py‑GC/MS, micro‑FTIR, LDIR, and electron microscopy) to allow comparability and dose-response assessment.
– Large, prospective population cohorts with baseline MNP exposure assessment and adjudicated cardiovascular outcomes to evaluate temporality and confounding.
– Mechanistic human studies (ex vivo vascular tissue exposure, controlled infusion models where ethical) and translational work linking particle attributes (size, shape, chemistry) to immune and endothelial responses.
– Focused evaluation of medical‑device shedding (CPB circuits, catheters, dialysis) with engineering solutions (materials, coatings, filters) and regulatory guidance to reduce iatrogenic exposure.
– Public‑health surveillance and exposure mitigation strategies targeting high‑exposure settings and vulnerable populations.
References
Zhang Y, Gao Q, Gao Q, Xu M, Fang N, Mu L, Han X, Yu H, Zhang S, Li Y, Gong Y. Microplastics and nanoplastics increase major adverse cardiac events in patients with myocardial infarction. J Hazard Mater. 2025 Jun 5;489:137624. doi: 10.1016/j.jhazmat.2025.137624. Epub 2025 Feb 19. PMID: 40007360.
Marfella R, Prattichizzo F, Sardu C, Fulgenzi G, Graciotti L, Spadoni T, D’Onofrio N, Scisciola L, La Grotta R, Frigé C, Pellegrini V, Municinò M, Siniscalchi M, Spinetti F, Vigliotti G, Vecchione C, Carrizzo A, Accarino G, Squillante A, Spaziano G, Mirra D, Esposito R, Altieri S, Falco G, Fenti A, Galoppo S, Canzano S, Sasso FC, Matacchione G, Olivieri F, Ferraraccio F, Panarese I, Paolisso P, Barbato E, Lubritto C, Balestrieri ML, Mauro C, Caballero AE, Rajagopalan S, Ceriello A, D’Agostino B, Iovino P, Paolisso G. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N Engl J Med. 2024 Mar 7;390(10):900-910. doi: 10.1056/NEJMoa2309822. PMID: 38446676; PMCID: PMC11009876. PDF (808.8 KB)
Wu Y, Chen Y, He R, Zhao T, Chen Z. Micronanoplastic exposure due to cardiopulmonary bypass in children: A prospective observational study. J Hazard Mater. 2025 Jun 5;489:137732. doi: 10.1016/j.jhazmat.2025.137732. Epub 2025 Feb 23. PMID: 40010211.