Background
Menopause, a natural biological milestone in women, often marks the decline of ovarian hormones, notably estrogens, which play crucial roles in cognition and neuroplasticity. Cognitive domains such as episodic memory, prospective memory, and executive functions tend to decline with advancing age, but these declines may be variably affected by menopause timing and hormone therapy. Menopausal hormone therapy (MHT), typically involving estrogens like estradiol (E2), aims to alleviate menopausal symptoms, yet its cognitive benefits remain debated. This uncertainty arises partly due to differences in hormone formulations and administration routes, which influence estrogen receptor (ER) activation in brain regions implicated in memory and executive function. Understanding how menopause age and different forms of estradiol-based MHT influence cognitive domains can inform personalized therapeutic strategies for aging women.
Study Design
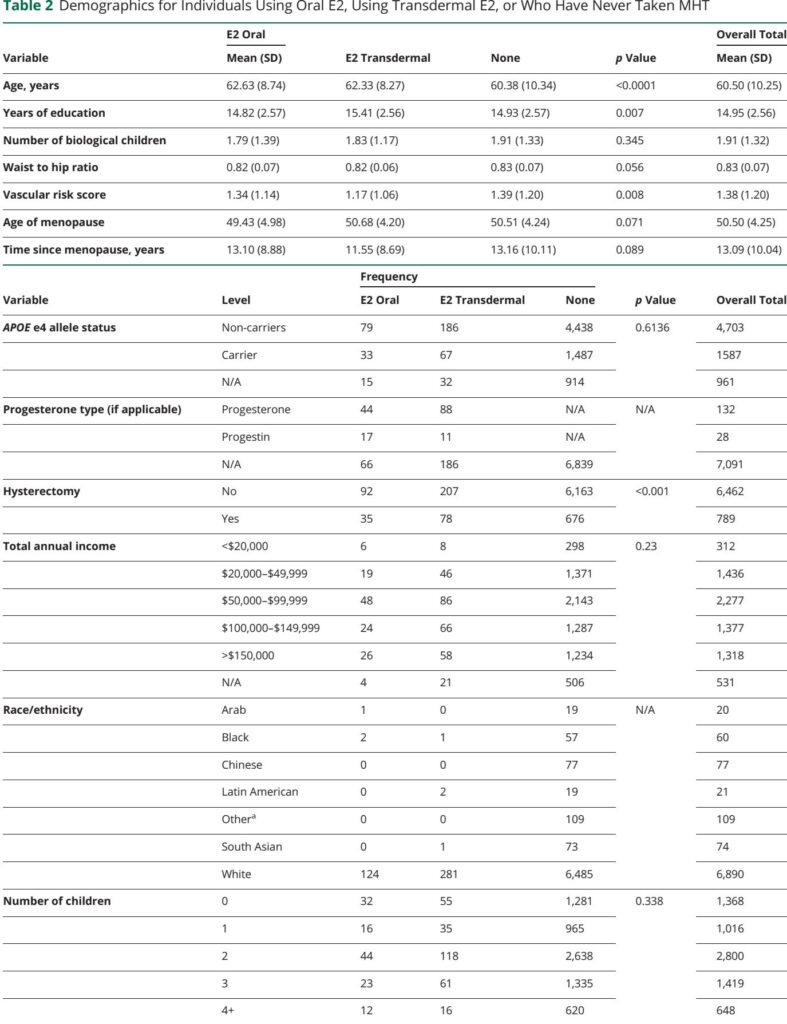
This cross-sectional observational study analyzed baseline data from 7,251 cognitively normal postmenopausal women (mean age 60.5 years) enrolled in the Canadian Longitudinal Study of Aging (CLSA). Participants were stratified by age at menopause and current use of estradiol-based MHT, categorized by administration route: oral or transdermal (including gels and vaginal preparations). Cognitive performance was assessed through composite scores derived from validated neuropsychological tests measuring episodic memory, prospective memory, and executive functions. Linear regression models adjusted for age, education, and vascular risk evaluated associations between menopause age, MHT route, and cognitive outcomes. Potential moderating effects of APOE e4 genotype and parity (number of children) were also examined.
Key Findings
Earlier menopause age was significantly associated with lower performance across all cognitive domains, including episodic memory, prospective memory, and executive function (all p < 0.001). Notably, the detrimental association between early menopause and executive function was more pronounced in women carrying the APOE e4 allele and in those with four or more children, suggesting genetic and reproductive history may exacerbate cognitive vulnerability.
Regarding MHT, the route of estradiol administration was differentially linked to memory domains. Transdermal estradiol use was significantly associated with higher episodic memory scores compared to no hormone therapy (p = 0.007, Cohen's d = 0.30), suggesting enhanced recall of past events. This effect likely stems from the avoidance of first-pass hepatic metabolism seen with oral estrogen, resulting in more stable plasma estradiol levels and potentially greater bioavailability for brain uptake.
Conversely, oral estradiol users exhibited better prospective memory—pertinent to remembering intentions and future tasks—compared with non-users (p = 0.015, Cohen's d = 0.28). Lower systemic doses or pharmacodynamics of oral administration may preferentially influence neural circuits underlying prospective memory. Neither administration route showed significant effects on executive function performance.
Importantly, neither APOE e4 carriage nor number of children modified the effects of estradiol therapy route on cognition. Sensitivity analyses excluding women with hysterectomy, progestogen use, or traumatic brain injury did not alter results, underscoring robustness.
Expert Commentary
These findings illuminate the nuanced interplay between menopause timing, estradiol therapy type, and cognitive domains. They reconcile inconsistencies in prior literature by demonstrating domain-specific cognitive benefits of estradiol based on administration route. Given increased estrogen receptor densities in frontal cortical regions post-menopause and consistent hippocampal engagement in episodic memory, the superiority of transdermal estradiol for episodic memory aligns with neurobiological expectations. Meanwhile, oral estradiol’s association with prospective memory suggests distinct pathways and hormonal sensitivities.
The study’s strengths include a large, well-characterized cohort and adjustment for confounders such as education and vascular risks, enhancing validity. Nevertheless, limitations encompass its cross-sectional design precluding causal inference, predominance of White, higher-income individuals limiting generalizability, and absence of detailed hormone therapy dosage and duration data. Additionally, self-reported menopause age and hormone use introduce potential recall bias.
Future longitudinal and randomized controlled trials are warranted to clarify causality and optimize MHT regimens tailored by cognitive endpoints.
Conclusion
The study substantiates that estradiol-based MHT’s cognitive effects vary by administration route and memory domain—transdermal estradiol preferentially benefits episodic memory, while oral estradiol enhances prospective memory, with neither significantly influencing executive function. Earlier menopause associates with cognitive impairment, particularly in genetically and reproductively predisposed women. These insights advocate for personalized approaches to menopausal hormone therapy, targeting specific cognitive outcomes to promote brain health in aging women.
Reference
Puri TA, Gravelsins LL, Alexander MW, McGovern AJ, Guterman PD, Rabin JS, Murphy KJ, Galea LAM. Association Between Menopause Age and Estradiol-Based Hormone Therapy With Cognitive Performance in Cognitively Normal Women in the CLSA. Neurology. 2025 Sep 23;105(6):e213995. doi: 10.1212/WNL.0000000000213995. Epub 2025 Aug 27. PMID: 40865026; PMCID: PMC12380479.