Introduction
Atrial fibrillation (AF) represents a prevalent cardiac arrhythmia affecting approximately one-third of individuals of European descent during their lifetime. Obesity is a well-established modifiable risk factor for AF, yet conventional measures such as body mass index (BMI) do not capture the nuances of fat distribution, particularly ectopic fat depots. Epicardial adipose tissue (EAT), a visceral fat compartment surrounding the myocardium, is hypothesized to influence AF pathogenesis through local paracrine mechanisms that promote inflammation and fibrosis. However, while EAT’s relationship with prevalent AF has been documented, its role as a predictor of incident AF in the general population remains less clear. This study aimed to clarify the association between EAT volume measured via computed tomography angiography (CTA) and the risk of developing new-onset AF in a large Danish cohort.
Study Design
This investigation was nested within the Copenhagen General Population Study (CGPS), a well-characterized population-based cohort from the Greater Copenhagen area. Between February 2010 and February 2012, participants aged 40 years or older with preserved renal function (serum creatinine <100 µmol/L) were invited for a cardiac CTA to quantify EAT volume. The study excluded individuals with a prior history of AF to focus on incident cases. The primary endpoint was new-onset AF, identified by the International Classification of Diseases, 10th Revision (ICD-10) code I48, sourced from the Danish National Patient Registry. The ICD-10 diagnosis of AF has a confirmed positive predictive value exceeding 92%, ensuring reliable case identification. Secondary endpoints included all-cause mortality obtained from national death registries.
Risk factors were comprehensively assessed via standardized questionnaires and clinical assessments, including blood pressure, BMI, glucose levels, spirometry, and lifestyle factors such as smoking and alcohol intake. Hypertension was defined as systolic/diastolic blood pressure ≥140/90 mmHg or use of relevant medication. Diabetes classification included self-reported diagnosis or plasma glucose ≥11.1 mmol/L. Chronic obstructive pulmonary disease (COPD) was defined by spirometric criteria (FEV1/FVC <70%). EAT volume was stratified into quartiles for analysis due to the lack of established cutoff values.
Key Findings
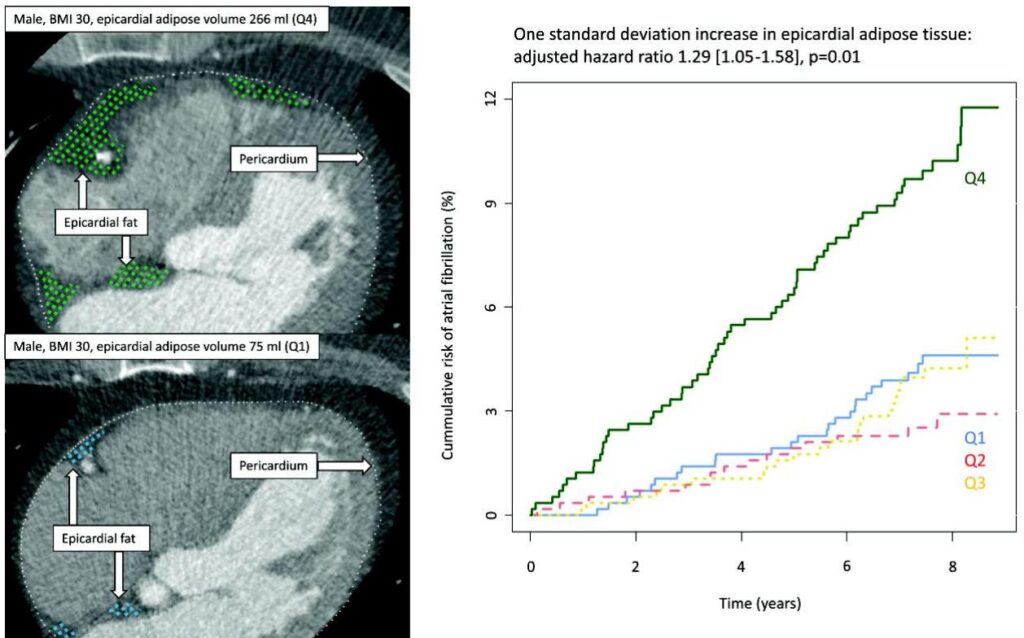
Among 2292 eligible participants (mean age 59.4 ± 10.3 years, 54% female), a median follow-up of 7.7 years yielded 123 incident AF cases (5.4%, incidence rate 7.2 per 1000 person-years). Baseline predictors of higher EAT volume included older age, male sex, elevated BMI, current smoking status, higher alcohol consumption, and presence of COPD.
Crucially, each standard deviation increase in EAT volume correlated with a 29% increased hazard of developing AF in multivariable Cox regression models adjusted for BMI, hypertension, smoking, alcohol use, diabetes, left atrial volume, COPD, and birth year (HR 1.29, 95% CI: 1.05–1.58, P = 0.01). Stratification across EAT quartiles demonstrated a graded increase in absolute AF risk, with the highest quartile (Q4) reaching an 11.4% cumulative incidence versus 4.6% in the lowest quartile (Q1) (Gray’s test P < 0.001). Notably, no significant differences in all-cause mortality were observed across EAT quartiles, underscoring the specificity of EAT’s association with AF risk rather than overall mortality.
Expert Commentary and Biological Plausibility
The study’s findings align with mechanistic evidence that EAT acts as an active endocrine organ secreting pro-inflammatory and fibrotic adipokines, termed adipo-fibrokines, which influence myocardial remodeling and electrical properties conducive to AF genesis. Compared with prior studies limited by shorter follow-up or less sensitive AF detection methods (e.g., single ECG recordings), this cohort utilized validated registry-based endpoints and a prolonged follow-up, enhancing the robustness and clinical relevance of the association.
Despite these strengths, several limitations warrant consideration. The possibility of selection bias exists as participants volunteering for a CTA scan may differ from the general population in health status and behaviors. Paroxysmal or asymptomatic AF episodes may have been undetected, potentially underestimating true incidence rates. As an observational study, causal inference remains limited, and residual confounding from physical activity levels or systemic inflammation is possible.
Conclusion
This Danish prospective cohort study substantiates that increased epicardial adipose tissue volume significantly elevates the risk of new-onset atrial fibrillation independently of BMI and other confounders. These results highlight the inadequacy of BMI alone in risk stratification and support the emerging paradigm where ectopic fat depots, notably EAT, contribute to arrhythmogenesis via localized inflammatory and fibrotic pathways.
Given that EAT is potentially modifiable through lifestyle interventions and pharmacotherapies, these findings open avenues for targeted AF prevention strategies. Future longitudinal and interventional studies incorporating serial EAT measurements are crucial to elucidate causality and assess whether therapeutic reduction in EAT can translate into lower AF incidence.
References
1. Van Gelder IC, Rienstra M, Bunting KV, Casado-Arroyo R, Caso V, Crijns HJGM, et al. 2024 ESC guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur Heart J 2024;45:3314–414. https://doi.org/10.1093/eurheartj/ehae176
2. Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 2007;153:907–17. https://doi.org/10.1016/j.ahj.2007.03.019
3. Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J 2015;36:795–805. https://doi.org/10.1093/eurheartj/eht099
4. Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 2017;9:eaal2658. https://doi.org/10.1126/scitranslmed.aal2658
5. Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT, et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol 2010;3:345–50. https://doi.org/10.1161/CIRCEP.109.912055
6. Rix TA, Riahi S, Overvad K, Lundbye-Christensen S, Schmidt EB, Joensen AM. Validity of the diagnoses atrial fibrillation and atrial flutter in a Danish patient registry. Scand Cardiovasc J 2012;46:149–53. https://doi.org/10.3109/14017431.2012.673728
7. Kühl JT, Lønborg J, Fuchs A, Andersen MJ, Vejlstrup N, Kelbæk H, et al. Assessment of left atrial volume and function: a comparative study between echocardiography, magnetic resonance imaging and multi slice computed tomography. Int J Cardiovasc Imaging 2012;28:1061–71. https://doi.org/10.1007/s10554-011-9930-2
8. Elming MB, Lønborg J, Rasmussen T, Kühl JT, Engstrøm T, Vejlstrup N, et al. Measurements of pericardial adipose tissue using contrast enhanced cardiac multidetector computed tomography—comparison with cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 2013;29:1401–7. https://doi.org/10.1007/s10554-013-0218-6
9. Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol 2022;19:593–606. https://doi.org/10.1038/s41569-022-00679-9
10. Mahabadi AA, Geisel MH, Lehmann N, Lammerding C, Kälsch H, Bauer M, et al. Association of computed tomography-derived left atrial size with major cardiovascular events in the general population: the Heinz Nixdorf Recall Study. Int J Cardiol 2014;174:318–23. https://doi.org/10.1016/j.ijcard.2014.04.068