Background
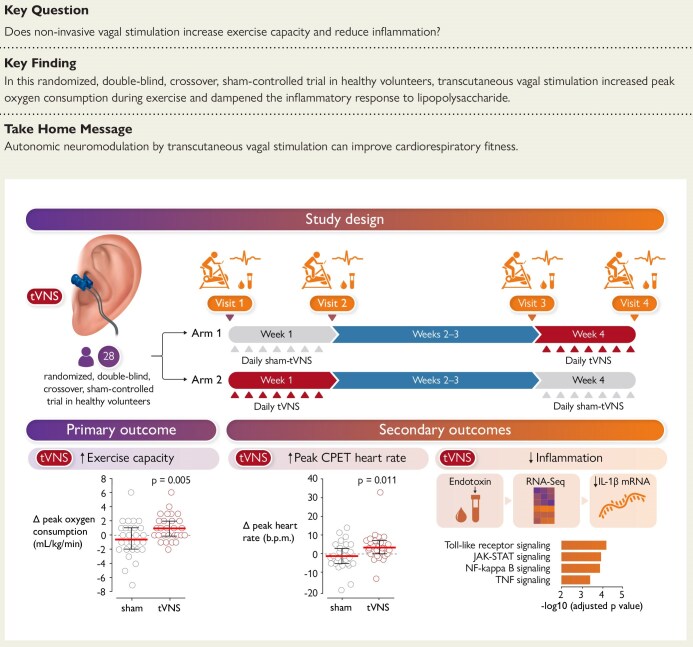
Exercise tolerance is a critical component of overall health and physical performance. Impaired exercise capacity, often associated with vagal parasympathetic dysfunction, is linked to reduced quality of life and increased risk of cardiovascular morbidity. Given that coordinated autonomic control is essential for optimal exercise performance, interventions targeting autonomic nervous system modulation are of growing interest. Non-invasive transcutaneous vagus nerve stimulation (tVNS) offers a promising approach to enhance parasympathetic activity without the risks associated with invasive procedures. However, evidence regarding its effectiveness in improving exercise capacity in healthy individuals remains limited. This study aimed to evaluate whether bilateral transcutaneous stimulation of auricular vagal innervation over seven days can enhance cardiorespiratory fitness and modulate inflammation in humans.
Study Design
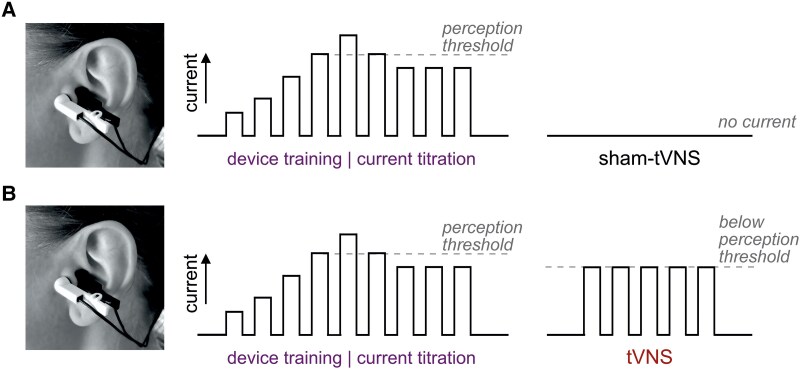
This was a single-centre, randomized, double-blind, sham-controlled, crossover trial conducted in 28 healthy volunteers, aged 20 to 63 years (mean age 34, 50% female). Participants underwent bilateral transcutaneous auricular vagus nerve stimulation or sham stimulation for 30 minutes daily over seven consecutive days. The primary endpoint was peak oxygen consumption (VO2peak) measured during progressive exercise to exhaustion. Secondary endpoints included peak work rate, cardiorespiratory parameters—such as respiratory and heart rate at peak exercise—and the whole blood inflammatory response to lipopolysaccharide stimulation ex vivo. Compliance was strictly monitored, with 100% adherence to interventions and assessments. The study maintained rigorous blinding procedures, as participants were unable to correctly identify their group allocation, and safety profiles were carefully recorded.
Key Findings
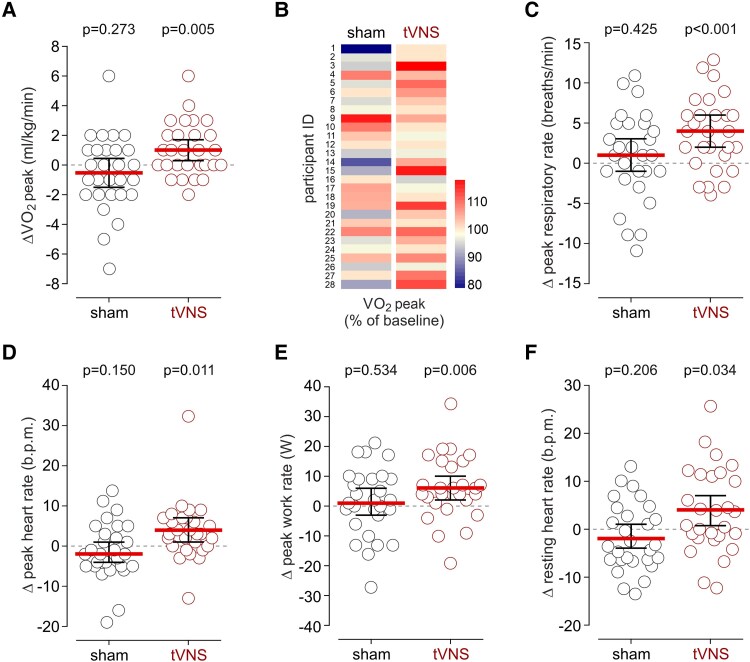
The mean baseline VO2peak was 32.9 mL/kg/min (95% CI: 29.4–36.4). After seven days of tVNS treatment, VO2peak increased significantly by 1.04 mL/kg/min (95% CI: 0.34–1.73; P = .005), corresponding to a 3.8% improvement (95% CI: 1.5–6.1). In contrast, sham-tVNS elicited no significant change (mean difference -0.54 mL/kg/min; 95% CI: -1.52 to 0.45; P = .273). This enhancement in oxygen uptake suggests improved aerobic capacity, a key determinant of exercise performance.
Secondary measures complemented these findings: tVNS increased respiratory rate at peak exercise by 4 breaths per minute (95% CI: 2–6; P < .001) and heart rate by 4 beats per minute (95% CI: 1–7; P = .011). Peak work rate rose by an average of 6 watts (95% CI: 2–10; P = .006) following active stimulation, whereas no meaningful changes occurred with sham treatment. These results collectively indicate augmented cardiorespiratory response and exercise performance attributable to tVNS.
Safety assessments revealed no significant adverse effects. The mean stimulation current was 5.5 ± 2 mA, with pre-specified side effects equally distributed between active and sham groups, and blinding integrity was maintained.
Additional exploratory analysis showed that tVNS attenuated blood inflammatory responses to lipopolysaccharide ex vivo, suggesting systemic anti-inflammatory effects that may underlie some benefits on exercise capacity, though details were supplementary and warrant further study.
Expert Commentary
The findings by Ackland et al. represent a noteworthy advancement in autonomic neuromodulation for enhancing exercise performance. The modest but statistically and clinically significant increase in VO2peak aligns with the known physiological importance of vagal tone in cardiovascular regulation and metabolic efficiency during exercise. The use of a double-blind crossover design and robust compliance strengthens the validity of the results. However, the study’s limitation includes the relatively small and healthy population, which may limit extrapolation to patients with autonomic dysfunction or chronic diseases where exercise capacity is impaired.
Mechanistically, tVNS may improve exercise performance through enhanced parasympathetic modulation, improved cardiopulmonary coupling, and reduced systemic inflammation. Future research should explore long-term effects, optimal stimulation protocols, and applications in clinical populations with impaired autonomic control or exercise intolerance.
Conclusion
This randomized controlled trial provides compelling evidence that non-invasive vagus nerve stimulation over seven days can safely and effectively improve cardiorespiratory fitness and exercise capacity in healthy adults. The procedure offers a low-cost, scalable intervention with minimal side effects, potentially benefiting broad populations seeking to enhance physical performance or rehabilitate exercise intolerance. These promising results warrant further exploration in larger, diverse cohorts and clinical contexts to fully establish tVNS as a therapeutic tool in exercise physiology and cardiovascular health.
References
Ackland GL, Patel ABU, Miller S, Gutierrez Del Arroyo A, Thirugnanasambanthar J, Ravindran JI, Schroth J, Boot J, Caton L, Mein CA, Abbott TEF, Gourine AV. Non-invasive vagus nerve stimulation and exercise capacity in healthy volunteers: a randomized trial. Eur Heart J. 2025 May 2;46(17):1634-1644. doi: 10.1093/eurheartj/ehaf037 IF: 35.6 Q1 . PMID: 39969124 IF: 35.6 Q1 ; PMCID: PMC7617618 IF: 35.6 Q1 .