Introduction
Respiratory syncytial virus (RSV) is a leading cause of respiratory illness in older adults and is increasingly recognized for its role in triggering cardiovascular (CV) complications, particularly in those with preexisting cardiovascular disease (CVD). Similar to influenza, RSV infection can precipitate events such as acute heart failure, myocardial infarction, and arrhythmias, largely mediated by inflammatory and prothrombotic processes. This makes prevention of RSV infection highly relevant not only for respiratory morbidity but also for reducing cardiovascular hospitalizations in this vulnerable population. The effectiveness of RSV vaccination on cardiovascular outcomes, however, has not been rigorously evaluated in randomized clinical trials until recently.
Study Design and Population
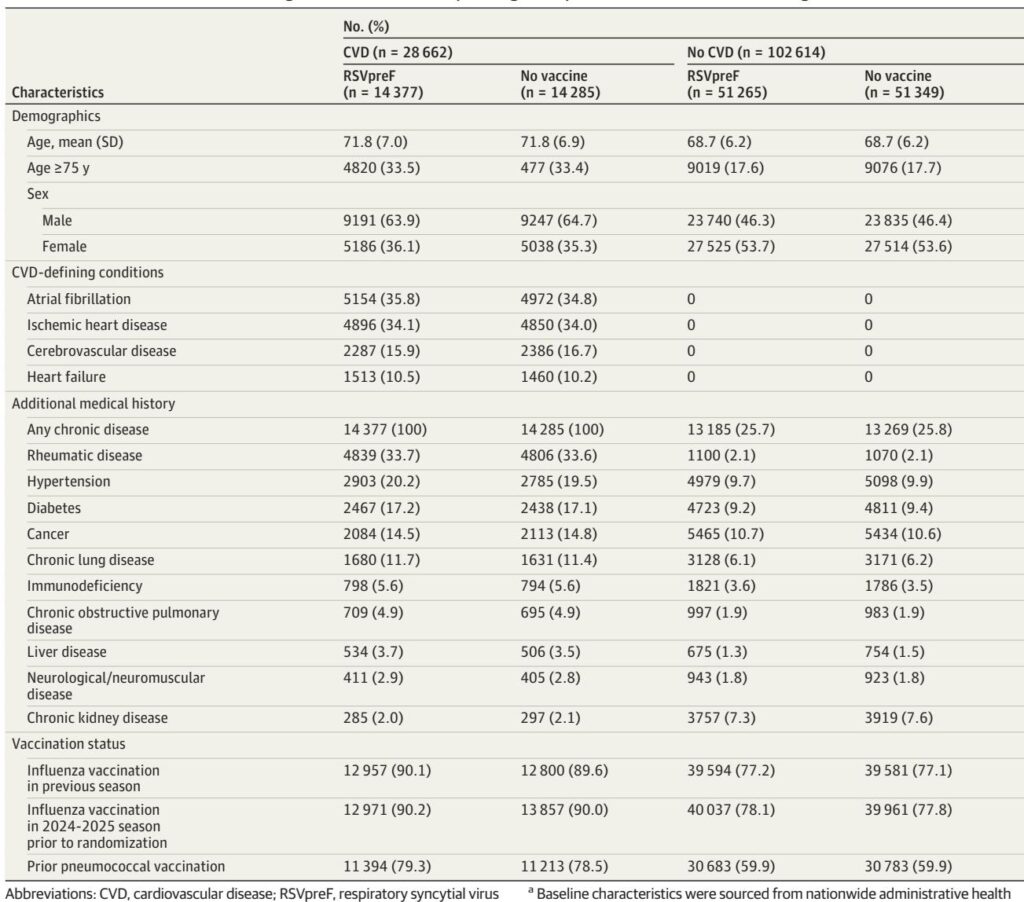
The DAN-RSV trial was a large pragmatic, open-label, randomized controlled study conducted in Denmark during the 2024-2025 winter season. More than 131,000 adults aged 60 years or older were randomized 1:1 to receive either a single dose of a bivalent RSV prefusion F protein (RSVpreF) vaccine or no vaccine. Participants were included irrespective of their comorbidity status, including those with and without preexisting cardiovascular disease. Follow-up extended from 14 days post scheduled vaccination visit until May 31, 2025, with data collected via national health registries ensuring comprehensive capture of hospitalization and mortality outcomes.
Endpoints and Statistical Methods
The primary endpoint for this analysis was hospitalization for any cardiorespiratory disease, a secondary endpoint within the larger DAN-RSV study. Exploratory cardiovascular endpoints included hospitalizations for heart failure, myocardial infarction, stroke, and atrial fibrillation. Vaccine effectiveness was estimated as 1 minus the incidence rate ratio, expressed as a percentage, with subgroup analyses stratified by baseline CVD status. Outcomes were adjudicated based on ICD-10 coding from health registries. Statistical analyses followed an intention-to-treat approach, with significance set at P<0.05.
Key Findings
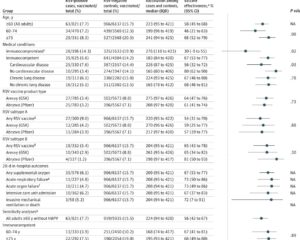
Among 131,276 participants (mean age 69.4; 50.3% male), 21.8% had preexisting CVD. RSVpreF vaccination significantly reduced all-cause cardiorespiratory hospitalization rates compared to no vaccine (26.3 vs. 29.2 events per 1000 person-years; vaccine effectiveness 9.9%, 95% CI 0.3%–18.7%; P=0.04). This benefit was observed consistently across cardiovascular and metabolic subgroups with no statistically significant interaction by baseline CVD status.
Although all-cause cardiovascular hospitalizations were numerically lower in the vaccinated group (16.4 vs 17.7 per 1000 person-years; vaccine effectiveness 7.4%, 95% CI -5.5% to 18.8%; P=0.24), individual cardiovascular outcomes such as myocardial infarction, heart failure hospitalization, stroke, and atrial fibrillation did not reach statistical significance. This may be due to the relatively low incidence rates and consequent limited statistical power for these exploratory endpoints.
Importantly, RSVpreF vaccination was highly effective at preventing RSV-related respiratory tract disease hospitalization, corroborating prior phase 3 trials. No significant safety concerns emerged, although a non-significant trend toward higher mortality in the vaccinated subgroup with CVD warrants further investigation with additional data.
Discussion
This prespecified analysis provides the first randomized evidence suggesting that RSV vaccination may lower the burden of hospitalizations from cardiorespiratory illness in adults aged 60 years or older. These findings parallel the well-established benefits of influenza vaccination, where reduction in respiratory infections also translates into reduced cardiovascular events and hospitalizations. The 9.9% relative reduction in all-cause cardiorespiratory hospitalizations, though modest, represents a meaningful public health impact given the high baseline incidence in this age group.
The underlying pathophysiology likely involves mitigation of RSV-triggered systemic inflammation and thrombosis, which can worsen existing cardiac conditions or precipitate new cardiovascular events. Since RSV testing is not routinely performed in cardiovascular admissions, some RSV-related cardiovascular events may remain unrecognized, potentially underestimating the vaccine effect on cardiovascular outcomes.
The trial’s innovative design—leveraging national registry data and digital recruitment—enabled robust, real-world effectiveness evaluation in a large, inclusive older adult population. While the open-label design and pragmatic nature of the trial may raise concerns about biases, the use of objective hospitalization endpoints and registry-based outcome adjudication mitigates these issues.
Limitations
Several caveats should be considered. The cardiovascular endpoints were exploratory and not statistically powered for definitive conclusions. Residual healthy volunteer bias cannot be excluded. Likewise, the modest sample size for specific CV subgroups and the predominantly Scandinavian cohort limit generalizability. No systematic RSV testing was instituted, which may have led to underascertainment of RSV-related cardiovascular events. Finally, the observed trend towards increased mortality in participants with CVD vaccinated with RSVpreF merits cautious interpretation pending further data.
Clinical Implications and Future Directions
The results underscore the broader benefits of RSV vaccination beyond respiratory symptom prevention, aligning with the experience from influenza vaccination in older adults. Vaccination strategies targeting RSV may reduce substantial cardiorespiratory morbidity and healthcare burden in an aging population with a high prevalence of chronic cardiovascular conditions. Further trials with longer follow-up and in diverse populations are warranted to clarify the cardiovascular protective effects and address mortality concerns. Routine integration of RSV vaccination into preventive care protocols for older adults, especially those with CVD, should be considered as evidence accrues.
Conclusion
The bivalent RSV prefusion F protein vaccine effectively reduces all-cause cardiorespiratory hospitalizations among adults aged 60 years or older, with potential, though not statistically significant, cardiovascular benefit. These findings support RSV vaccination as an important preventive measure to mitigate infection-triggered cardiovascular complications and improve care in older adults with or without preexisting cardiovascular disease.
Reference:
Lassen MCH, Johansen ND, Christensen SH, Aliabadi N, Skaarup KG, Modin D, Claggett BL, Larsen CS, Larsen L, Wiese L, Dalager-Pedersen M, Lindholm MG, Jensen AMR, Dons M, Bernholm KF, Davidovski FS, Duus LS, Ottosen CI, Nielsen AB, Borchsenius JH, Espersen C, Köse G, Fussing FH, Køber L, Solomon SD, Jensen JUS, Martel CJ, Gessner BD, Schwarz C, Gonzalez E, Skovdal M, Moulton LH, Zhang P, Begier E, Biering-Sørensen T. RSV Prefusion F Vaccine for Prevention of Hospitalization in Older Adults. N Engl J Med. 2026 Jan 8;394(2):138-151. doi: 10.1056/NEJMoa2509810 IF: 78.5 Q1 . Epub 2025 Aug 30. PMID: 40888695 IF: 78.5 Q1 .