Highlight
– Sequential administration of parecoxib followed by imrecoxib significantly reduced the occurrence of severe acute pancreatitis (SAP) by 20.7% compared to placebo.
– The duration of persistent organ failure (OF) was shortened by two days in patients treated with COX-2 inhibitors.
– Local complications and systemic inflammatory mediators were notably less frequent and reduced, respectively, after COX-2-Is treatment.
– The 30-day mortality rate dropped from 8.6% in the placebo group to 3.4% with sequential COX-2 inhibitor therapy, with a similar adverse event profile between treatment groups.
Study Background and Disease Burden
Severe acute pancreatitis (SAP) is a critical clinical syndrome characterized by the sudden inflammation of the pancreas leading to systemic inflammatory response and multi-organ failure. Despite advances in supportive care, effective pharmacological therapies targeting organ failure—the primary determinant of morbidity and mortality in SAP—remain lacking. The severe inflammatory cascade underlying SAP pathophysiology involves cyclooxygenase-2 (COX-2) mediated pathways contributing to tissue injury and systemic complications. Given the absence of targeted therapies to mitigate organ failure, this study evaluated the efficacy and safety of sequential COX-2 inhibitors, parecoxib and imrecoxib, aimed at attenuating inflammation and improving clinical outcomes in SAP.
Study Design
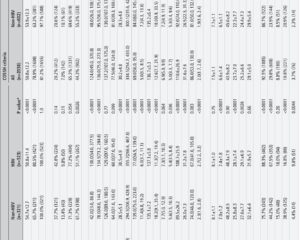
This was a multicentre, double-blind, randomized, placebo-controlled, investigator-initiated trial involving 348 patients aged 18–75 years diagnosed with acute pancreatitis within one week of symptom onset. Eligibility criteria included patients with an Acute Physiology and Chronic Health Evaluation II (APACHE II) score ≥7 or modified Marshall score ≥2, indicating moderate to severe disease and early signs of organ failure risk. Patients were allocated 1:1 to receive either the COX-2 inhibitor treatment sequentially—intravenous parecoxib followed by oral imrecoxib—or matching placebo controls.
The primary endpoints included the occurrence of SAP and duration of persistent organ failure. Secondary endpoints encompassed the frequency of local complications such as pancreatic necrosis or fluid collections, serum inflammatory mediator levels (including cytokines), 30-day mortality, and adverse event incidence. Stratified analyses examined outcomes relative to timing from symptom onset (48 hours) at enrollment.
Key Findings
Parecoxib and imrecoxib combination treatment demonstrated significant clinical benefit over placebo:
1. SAP occurrence was reduced from 77.6% in the placebo group to 61.5% in the COX-2-Is group (absolute reduction 20.7%, p=0.001). Notably, early enrollment (<48 hours) saw a more pronounced SAP risk reduction (23.8%, p=0.001) compared with later enrollment (8.5%, p=0.202), indicating a time-sensitive therapeutic window.
2. Duration of persistent organ failure in SAP patients treated with COX-2-Is was shortened by two days overall (p<0.001). Early treatment patients experienced a three-day reduction (p=0.001), while later treated patients had a two-day reduction (p=0.010).
3. Local complication rates were significantly lower in the treatment group (33.7%) than placebo (49.1%, p=0.004), suggesting a reduction in pancreatic and peripancreatic tissue injury.
4. Serum inflammatory mediators, reflective of systemic inflammation, were significantly decreased in the treatment arm, supporting the anti-inflammatory action of COX-2 inhibition.
5. Thirty-day mortality was reduced from 8.6% in the placebo group to 3.4% with COX-2-Is (p<0.05), highlighting meaningful survival benefit.
6. Safety profiles were comparable, with similar adverse event rates between placebo and treatment groups, indicating good tolerability.
These data affirm the clinical efficacy and safety of parecoxib sequential with imrecoxib in mitigating SAP severity and sequelae by targeting systemic inflammation and reducing organ dysfunction burden.
Expert Commentary
This landmark randomized trial is the first to establish COX-2 inhibition as an effective pharmacological intervention for SAP, a condition historically devoid of targeted therapies beyond supportive care. The robust reduction in SAP incidence and organ failure duration underscores the therapeutic potential of interrupting inflammatory pathways central to pancreatitis progression. The sequential approach—starting with parecoxib for rapid COX-2 blockade followed by oral imrecoxib consolidation—enables sustained anti-inflammatory effects with practical application in clinical settings.
However, limitations include the study’s restriction to patients within 7 days of onset, potentially limiting generalizability to late presenters. Longer-term outcomes beyond 30 days were not assessed, and further exploration into mechanistic biomarkers could deepen understanding of treatment response heterogeneity. Importantly, the timing of intervention impacts efficacy, underscoring the need for early diagnosis and initiation in SAP management.
Integration of this therapeutic strategy into clinical guidelines could transform the SAP treatment landscape, shifting from purely supportive to disease-modifying care. Ongoing research should explore combination therapies and further delineate patient subgroups most likely to benefit.
Conclusion
The multicentre, double-blind, placebo-controlled trial by Huang et al. confirms that sequential administration of the COX-2 inhibitors parecoxib and imrecoxib is an effective, well-tolerated approach to reduce the occurrence and severity of severe acute pancreatitis. By suppressing systemic inflammatory responses, this regimen shortens organ failure duration, decreases local complications, and lowers mortality rates, addressing a critical unmet need in SAP management. Early treatment initiation appears to enhance therapeutic efficacy. These findings have important implications for the development of targeted pharmacotherapy in SAP, offering new hope for reducing its high morbidity and mortality.
References
Huang L, Feng Z, Yang W, Zhu Y, Li J, Huang L, Wang R, Peng L, He M, Tang Y, Chen P, Lan C, Zhou X, Zhou L, Ye C, Zhang L, Jiang J, Ye Y, Wang R, He Y, Liu Y, Gong H, Xiong H, Xia L, Xu H, Zhang B, Tu R, Du C, Cui L, Gao J, Huang Z, Tang C. Parecoxib sequential with imrecoxib for occurrence and remission of severe acute pancreatitis: a multicentre, double-blind, randomised, placebo-controlled trial. Gut. 2025 Aug 7;74(9):1467-1475. doi: 10.1136/gutjnl-2024-334038. PMID: 40301118.