Introduction
Atrial fibrillation (AF) poses significant public health challenges by increasing the risk of stroke and systemic embolism, necessitating effective management strategies. Catheter ablation has emerged as a cornerstone intervention aimed at restoring and maintaining sinus rhythm, improving symptoms and quality of life. However, optimal long-term anticoagulation management following successful ablation remains unsettled. Existing guidelines advocate for continuous oral anticoagulant (OAC) therapy in patients with elevated stroke risk, yet robust randomized clinical data supporting this approach are limited.
The ALONE-AF trial addresses a critical clinical question: can discontinuing OAC therapy after successful AF ablation, in patients without documented atrial arrhythmia recurrence, provide superior clinical outcomes compared to continued anticoagulation? This investigation offers pivotal insights into balancing thromboembolic prevention with bleeding risk in this patient population.
Methods
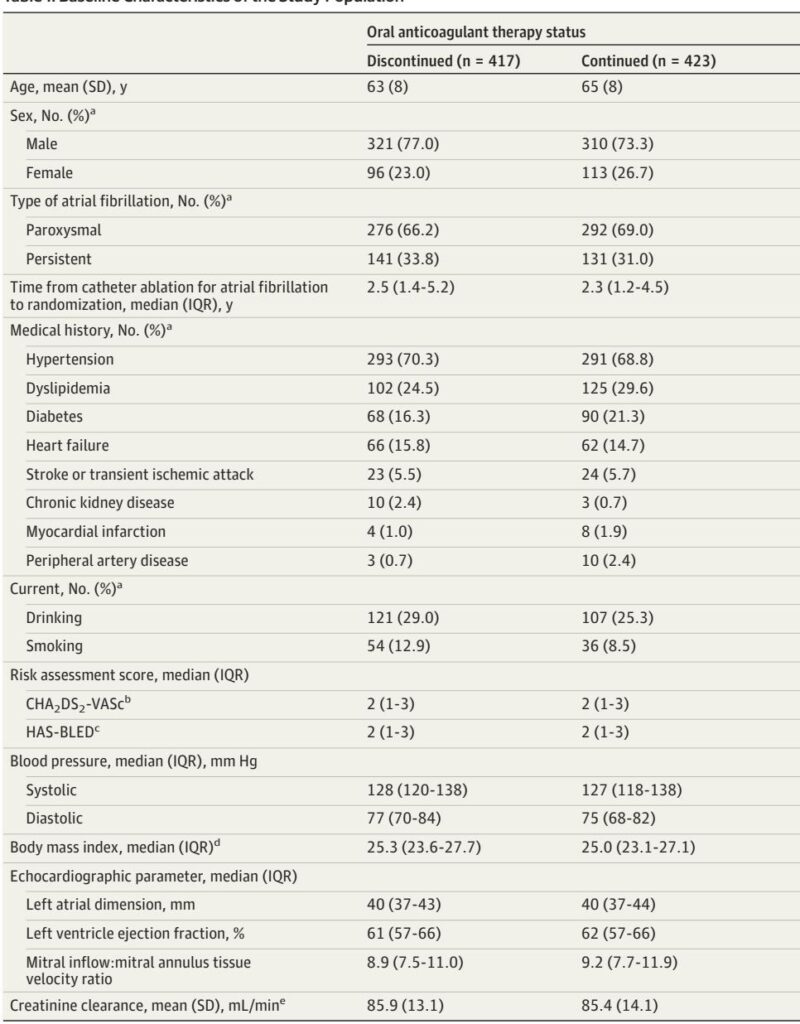
The ALONE-AF study was a multicenter, open-label, superiority randomized clinical trial conducted across 18 South Korean hospitals, enrolling 840 adult patients (age 19–80 years) from July 2020 to March 2023. Eligibility criteria included prior AF catheter ablation with no atrial arrhythmia recurrence documented for at least one year, and possessing at least one non–sex-related stroke risk factor defined by the CHA2DS2-VASc score (men 2 1, women 2 2).
Participants were randomized 1:1 to either discontinue oral anticoagulant therapy (intervention group, n=417) or continue OAC therapy with direct oral anticoagulants (DOACs), primarily apixaban or rivaroxaban, (control group, n=423). Dosing adjustments followed established clinical criteria based on age, body weight, and renal function.
Primary outcome was the composite of stroke, systemic embolism, and major bleeding over a two-year follow-up. Secondary outcomes included individual components of the primary endpoint, transient ischemic attack, clinically relevant nonmajor bleeding, myocardial infarction, all-cause mortality, and hospitalizations.
All patients underwent routine ECG and periodic 24- to 72-hour Holter monitoring every six months, supplemented by symptom-guided assessments to detect arrhythmia recurrence. Those with confirmed recurrence or undergoing repeat ablation were censored and managed according to thromboembolic risk guidelines.
The study hypothesis posited that OAC discontinuation would reduce the primary composite endpoint primarily through decreased bleeding events without significantly increasing ischemic complications. The sample size was powered at 80% with an anticipated 7% dropout and an expected absolute risk reduction of 5.0% at two years.
Results
Baseline demographics were balanced between groups, with a mean patient age of 64 years, 24.9% women, and mean CHA2DS2-VASc score of 2.1. Paroxysmal AF was present in 67.6%, and the average interval from catheter ablation to randomization was 3.6 years.
At two-year follow-up, the primary composite outcome occurred in 0.3% of patients in the OAC discontinuation group versus 2.2% in the continued OAC group (absolute difference 2 percentage points; 95% CI, 2 to 0.3; P=0.02). Importantly, this reduction was driven by major bleeding events, which occurred exclusively in the continued OAC group (1.4%), while ischemic stroke incidence was similarly low in both groups (0.3% vs 0.8%).
Subgroup analyses including patients with high stroke risk (CHA2DS2-VASc 2 4) demonstrated consistent benefits of OAC discontinuation without increased ischemic events. No deaths or myocardial infarctions were reported.
The incidence of atrial arrhythmia recurrence post-randomization was approximately 9%, with OAC therapy reinitiated accordingly. Adherence to randomized treatment was high, with minimal crossovers.
Expert Commentary
This trial challenges the prevailing paradigm mandating indefinite anticoagulation in all patients at risk post-AF ablation. The findings underscore that a carefully selected subset—patients free from atrial arrhythmia recurrence for at least one year and intermediate to high stroke risk—may safely discontinue anticoagulation, reducing bleeding complications without increasing thromboembolic events.
The low ischemic event rates observed likely reflect the reduced AF burden post-ablation, corroborated by prior observational studies and mechanistic insights into stroke risk modulation.
Regular rhythm monitoring is paramount for safe OAC discontinuation due to the poor correlation between AF symptoms and arrhythmia episodes. While continuous monitoring remains the gold standard, practical alternatives such as intermittent Holter and symptom-driven ECG monitoring were effectively utilized in ALONE-AF.
Notably, although the open-label design and predominantly East Asian cohort may limit generalizability, the rigor of adjudication and multicenter involvement enhance study validity. The use of a composite primary endpoint emphasizing net clinical benefit is justified given the competing risks of bleeding and thrombosis.
These findings are aligned with emerging evidence questioning universal OAC continuation post-ablation and highlight personalized anticoagulation strategies.
Conclusions
The ALONE-AF randomized clinical trial establishes that, among patients without documented atrial arrhythmia recurrence for at least one year after catheter ablation of AF, discontinuation of oral anticoagulant therapy significantly reduces the composite risk of stroke, systemic embolism, and major bleeding compared to continued DOAC therapy. This benefit chiefly arises from fewer major bleeding events without a concomitant rise in ischemic complications.
These data advocate for a paradigm shift toward individualized cessation of anticoagulation in suitable patients under careful rhythm surveillance, potentially sparing many from bleeding risks associated with prolonged anticoagulation. Further studies may delineate optimal rhythm monitoring strategies and extend validation to diverse populations.