Introduction
Respiratory syncytial virus (RSV) is a leading cause of seasonal respiratory infections, particularly affecting older adults and those with chronic cardiopulmonary conditions. Each year in the United States, RSV accounts for an estimated 100,000 to 150,000 hospitalizations and 4,000 to 8,000 deaths among adults aged 60 years and older. In response to this significant disease burden, three RSV vaccines targeting the RSV prefusion F protein have been licensed for adults aged 60 years or older. Two protein subunit vaccines, Arexvy (GSK) and Abrysvo (Pfizer), were introduced in 2023, with an mRNA vaccine, mRESVIA (Moderna), approved in 2024.
Initial recommendations advised a single dose for adults aged 60+ based on clinical judgment, evolving by June 2024 into a universal recommendation for adults 75+ and a risk-based approach for 60 to 74-year-olds at increased risk. Duration of protection and optimal revaccination intervals remain unclear, highlighting a critical need to evaluate vaccine effectiveness (VE) in real-world settings beyond clinical trials.
Study Design and Population
This multicenter, test-negative, case-control study enrolled 6,958 adults aged 60 years or older hospitalized with acute respiratory illness at 26 hospitals across 20 US states during two consecutive RSV seasons (October 2023–March 2024 and October 2024–April 2025). Patients were included if respiratory virus testing occurred within 10 days of illness onset; RSV-positive cases were identified by PCR testing, while controls tested negative for RSV, SARS-CoV-2, and influenza. Those co-infected with other respiratory viruses were excluded to minimize confounding related to vaccine behaviors.
Vaccination status was rigorously ascertained via electronic health records, immunization registries, and patient self-report, categorized into vaccinated (single dose at least 14 days before illness onset) and unvaccinated groups. Analyses further stratified participants by season of vaccination (same vs prior) relative to illness.
Key Findings
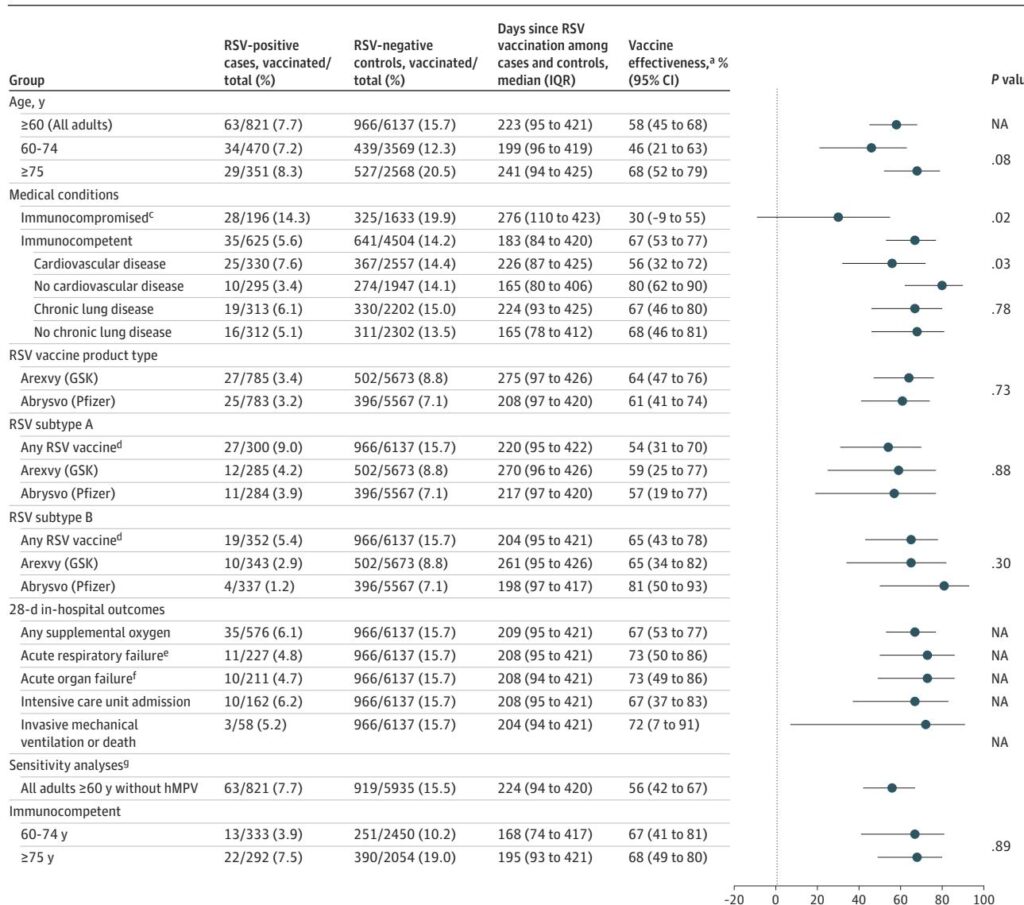
Overall, RSV vaccination conferred a 58% reduction (95% CI, 45%–68%) in odds of RSV-associated hospitalization over two seasons, with median follow-up of approximately 223 days post-vaccination. Vaccine effectiveness was higher when vaccination was received in the same season as illness onset (69%; 95% CI, 52%–81%) compared to prior season vaccination (48%; 95% CI, 27%–63%), although this difference was not statistically significant (P=0.06).
Subgroup analyses revealed:
– Adults aged 75 years or older had numerically higher VE (68%; 95% CI, 52%–79%) than those 60–74 years (46%; 95% CI, 21%–63%), though this did not reach statistical significance (P=0.08).
– Immunocompromised patients exhibited significantly lower vaccine effectiveness (30%; 95% CI, –9% to 55%) compared to immunocompetent adults (67%; 95% CI, 53%–77%; P=0.02).
– Individuals with cardiovascular disease also demonstrated reduced VE (56%; 95% CI, 32%–72%) relative to those without cardiovascular disease (80%; 95% CI, 62%–90%; P=0.03).
– No significant differences in VE were observed based on chronic lung disease status or RSV subtype (A versus B).
– Effectiveness estimates were similar for the two major vaccine products used, Arexvy and Abrysvo.
Furthermore, RSV vaccination showed substantial protection against severe in-hospital outcomes such as acute respiratory failure (VE 73%), organ failure (VE 73%), ICU admission (VE 67%), and invasive mechanical ventilation or death (VE 72%).
Clinical and Immunological Considerations
The study’s findings align with earlier clinical trials reporting efficacy of RSV vaccines against lower respiratory tract disease, albeit with novel insights into real-world effectiveness against hospitalization and severe outcomes. Notably, the reduced VE in immunocompromised adults likely reflects diminished immune responsiveness post-vaccination, as supported by immunogenicity studies showing lower neutralizing antibody titers in transplant recipients and those undergoing immunosuppressive therapy.
Similarly, the decreased VE among patients with cardiovascular disease—majority with heart failure—may be influenced by underlying proinflammatory states that compromise immune defense mechanisms, increasing susceptibility to severe RSV disease and potentially attenuating vaccine response.
The durability of protection, though showing some suggestion of waning with prior-season vaccination, requires further follow-up to refine optimal revaccination intervals. Early immunogenicity data indicate revaccination boosts antibody levels but may not restore them to initial peak responses, while clinical trial data suggest limited added benefit when revaccinating at 12 months.
Study Limitations
This study has important limitations. The relatively low vaccination coverage among eligible controls (15.7%) introduces potential bias, as early adopters may differ systematically from the general population, possibly confounding vaccine effectiveness estimates due to differences in health behaviors or comorbidities. The sample size limited subgroup analyses by specific types of immunosuppression. Additionally, recent prior RSV infection, which might confer natural immunity, was not accounted for.
Conclusions
In a large, geographically diverse, and clinically representative cohort, a single dose of RSV vaccine was effective in reducing RSV-associated hospitalizations and severe clinical outcomes in adults aged 60 years and older across two seasons. However, effectiveness is attenuated in immunocompromised individuals and those with cardiovascular disease, underscoring the need for tailored vaccination strategies such as earlier revaccination or adjunctive preventive measures for these vulnerable populations.
As vaccine recommendations expand to include adults aged 50 to 59 at increased risk of severe RSV disease, continuous surveillance of vaccine effectiveness and durability will be critical to inform clinical guidelines and optimize preventive strategies against RSV.
References
Surie D, Self WH, Yuengling KA, Lauring AS, Zhu Y, Safdar B, Ginde AA, Simon SJ, Peltan ID, Brown SM, Gaglani M, Ghamande S, Columbus C, Mohr NM, Gibbs KW, Hager DN, Prekker M, Gong MN, Mohamed A, Johnson NJ, Steingrub JS, Khan A, Duggal A, Gordon AJ, Qadir N, Chang SY, Mallow C, Felzer JR, Kwon JH, Exline MC, Vaughn IA, Ramesh M, Papalambros L, Mosier JM, Harris ES, Baughman A, Cornelison SA, Blair PW, Johnson CA, Lewis NM, Ellington S, Grijalva CG, Talbot HK, Casey JD, Halasa N, Chappell JD, Rutkowski RE, Ma KC, Dawood FS; Investigating Respiratory Viruses in the Acutely Ill (IVY) Network. RSV Vaccine Effectiveness Against Hospitalization Among US Adults Aged 60 Years or Older During 2 Seasons. JAMA. 2025 Aug 30:e2515896. doi: 10.1001/jama.2025.15896. Epub ahead of print. PMID: 40884491; PMCID: PMC12398767.