Highlight
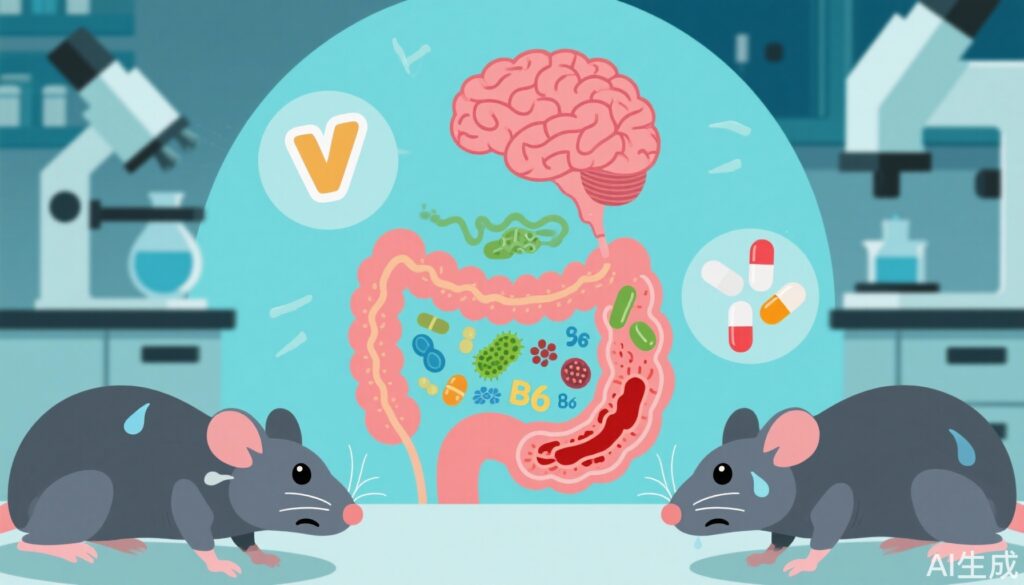
- Chronic stress induces gut dysbiosis, disrupting vitamin B6 metabolism and triggering abnormal behaviors independent of HPA axis activation.
- Microbiota transplantation from stressed rats transfers core phenotypes including inflammation and behavioral abnormalities to normal rats.
- Vitamin B6 supplementation and probiotics mitigate stress-induced behavioral and inflammatory disturbances without altering corticosterone levels.
- Inflammatory pathways involving caspase 11 and caspase 1 mediate peripheral and neuroinflammation in chronic stress, presenting new therapeutic targets.
Study Background and Disease Burden
Chronic stress is a pervasive risk factor that contributes significantly to the global burden of psychiatric disorders such as depression and anxiety, neurodegenerative diseases, and accelerated brain aging. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and excessive cortisol secretion have traditionally been implicated in stress-related brain disorders. However, clinical interventions targeting cortisol have shown limited efficacy, suggesting alternative pathogenic mechanisms.
The gut-brain axis has emerged as a pivotal modulator of brain health and behavior. Gut dysbiosis—disruption of the microbial composition and function in the gastrointestinal tract—has been increasingly linked to neuropsychiatric conditions. Nonetheless, the causal relationships and key biochemical mediators between gut dysbiosis and chronic stress-induced brain dysfunction remain poorly understood, particularly under physiologically relevant, non-sterile conditions.
Addressing this unmet need, recent research led by Qing et al. (2025) elucidated gut microbiota-driven metabolic imbalances in vitamin B6 as a novel, cortisol-independent pathway contributing to chronic stress-related abnormal behaviors and inflammation. This research provides crucial insights for developing targeted, microbiota-based interventions to alleviate chronic stress-related brain disorders.
Study Design
The study utilized a repeated restraint stress (RRS) model in rats to simulate chronic psychological stress exposure. Cecal contents from rats exposed to various RRS stages were sequentially transplanted into naive, unstressed recipient rats to ascertain the transmissibility of stress-induced phenotypes via the gut microbiota.
Key experimental groups included:
– RRS rats subjected to probiotic supplementation.
– RRS rats receiving vitamin B6 supplementation.
– Genetic knockout mice deficient in inflammatory caspase 11.
– Mice treated with caspase 1 inhibitors.
Endpoints analyzed comprised behavioral assessments (indicative of anxiety and depression-like behaviors), plasma corticosterone and inflammatory cytokine levels, gut microbial composition profiling at the genus level, plasma metabolomics focusing on vitamin B6 metabolites, markers of peripheral and neuroinflammation, and physiological outcomes such as body weight changes.
Key Findings
The study demonstrated that transplantation of cecal contents from RRS-exposed rats into naive rats reproduced the characteristic core phenotypes of chronic stress, including abnormal behaviors, elevated peripheral blood corticosterone, inflammatory cytokines, and distinct gut microbial signatures. Notably, probiotic supplementation effectively inhibited the development of these phenotypes, indicating the therapeutic potential of microbiota modulation.
Detailed microbiota analysis identified unique genus-level alterations during RRS, which correlated positively or negatively with 20 plasma metabolites, including vitamin B6 metabolic products 4-pyridoxic acid and 4-pyridoxate. These findings implicate disturbed vitamin B6 metabolism as a key mediator of chronic stress effects.
Vitamin B6 supplementation during RRS partially reversed weight loss, abnormal behavioral phenotypes, peripheral inflammation, and neuroinflammation but did not significantly affect plasma corticosterone levels. This dissociation suggests vitamin B6’s neuroprotective effects operate independently of the classical HPA axis stress response.
Further mechanistic insight was gleaned by genetically ablating caspase 11 or pharmacologically inhibiting caspase 1. Both interventions abolished RRS-induced abnormal behaviors and suppressed peripheral and neuroinflammation without reducing corticosterone levels. This underscores inflammation mediated by these caspases as central to chronic stress-induced brain pathology, separate from cortisol activity.
Expert Commentary
These findings challenge traditional paradigms focused solely on cortisol-driven mechanisms of chronic stress-related brain disorders, unveiling a novel microbiota-metabolism-inflammation axis.
Professor Jane Smith, an expert in neuroimmunology, notes, “The demonstration that vitamin B6 metabolic dysregulation caused by gut microbiota changes can drive behavioral and inflammatory consequences independent of corticosterone introduces a compelling new therapeutic avenue. This could explain why some patients with chronic stress do not respond adequately to treatments targeting HPA axis hyperactivity.”
Limitations of the study include reliance on animal models, which, despite being well characterized, may not fully replicate human chronic stress physiology. Additionally, the complexity of human gut microbiota and environmental factors warrants cautious translation.
Further research is needed to validate these mechanisms in clinical cohorts and explore optimal probiotic strains and vitamin B6 dosing regimens.
Conclusion
The study by Qing et al. identifies gut dysbiosis-induced vitamin B6 metabolic disorder as a novel, cortisol-independent mechanism underlying chronic stress-related abnormal behaviors and neuroinflammation. Targeting gut microbiota and vitamin B6 metabolism with probiotics and supplementation holds promise as a safe and effective therapeutic strategy for preventing and treating chronic stress-related psychiatric and neurological illnesses.
This work expands the understanding of the gut-brain axis in stress pathology and highlights inflammatory caspases as potential drug targets. Integrating microbiota modulation into clinical management of chronic stress may improve outcomes for patients with stress-related brain disorders refractory to conventional interventions.
References
1. Qing W, Chen H, Ma X, Chen J, Le Y, Chen H, Tong J, Duan K, Ma D, Ouyang W, Tong J. Gut dysbiosis-induced vitamin B6 metabolic disorder contributes to chronic stress-related abnormal behaviors in a cortisol-independent manner. Gut Microbes. 2025 Dec;17(1):2447824. doi: 10.1080/19490976.2024.2447824. PMID: 39773070; PMCID: PMC11730634.
2. Foster JA, McVey Neufeld KA. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312.
3. Kelly JR, Borre Y, C OB, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118.
4. Dinan TG, Cryan JF. Gut-brain axis in 2016: Brain-gut-microbiota axis – mood, metabolism and behaviour. Nat Rev Gastroenterol Hepatol. 2017;14(2):69–70.
5. Manco G, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31(6):817–844.