Highlight
- Intracerebral hemorrhage (ICH) is a life-threatening stroke subtype often complicated by secondary white matter injury that worsens prognosis.
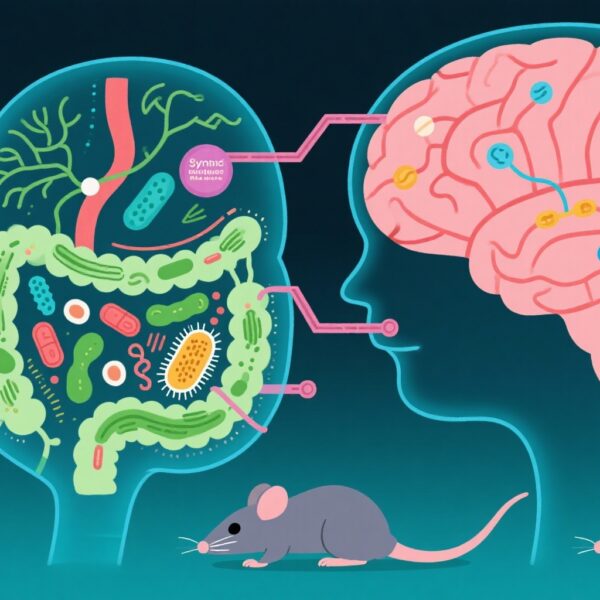
- Bidirectional communication through the gut microbiota-brain axis influences inflammation and recovery after ICH, with dysbiosis being a key contributor.
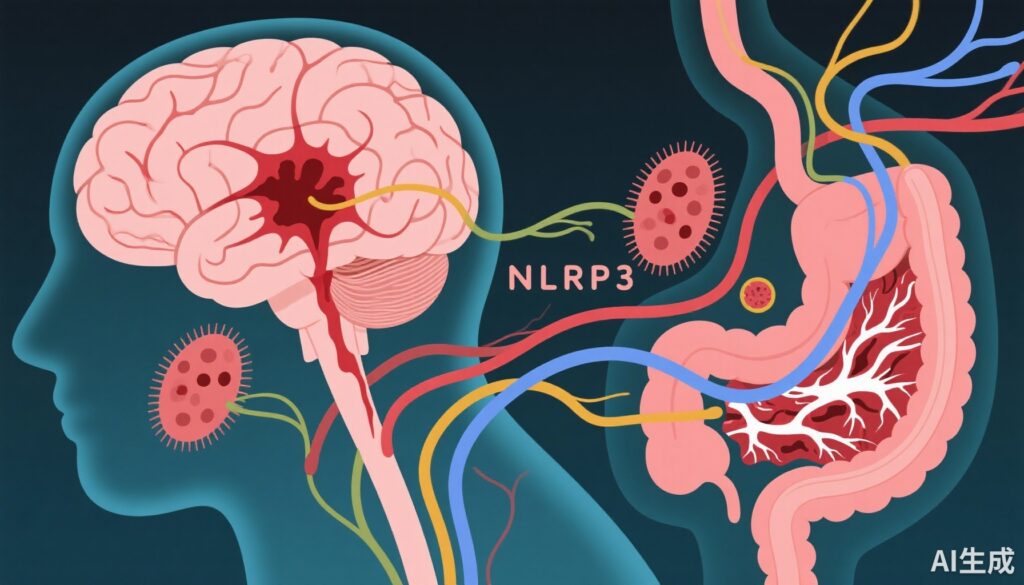
- The NLRP3 inflammasome is a pivotal mediator linking gut microbiota disturbances to neuroinflammation and white matter injury.
- Targeting the gut microbiota-brain axis and inhibiting NLRP3 inflammasome offer promising therapeutic strategies for improving outcomes after ICH.
Study Background and Disease Burden
Intracerebral hemorrhage (ICH) accounts for 10-15% of all strokes and represents the most devastating stroke subtype due to high rates of mortality and long-term disability. Secondary injury mechanisms, especially white matter injury (WMI) surrounding the hematoma, significantly contribute to poor functional outcomes by impairing neuronal connectivity and repair processes. Despite advances in acute management, effective therapies targeting secondary brain injury after ICH remain limited.
In recent years, increasing evidence supports a crucial role of the gut microbiota-brain axis—a complex bidirectional system connecting the gastrointestinal tract and central nervous system— in influencing stroke pathophysiology and recovery. This axis involves neural, immune, and metabolic pathways that modulate neuroinflammation and neuroregeneration.
One critical molecular player associated with inflammation after ICH is the NLRP3 (NACHT, LRR, and pyrin domain-containing protein 3) inflammasome. Activation of the NLRP3 inflammasome triggers release of proinflammatory cytokines such as IL-1β and IL-18, exacerbating neuroinflammatory cascades that contribute to BBB disruption and WMI. Understanding how gut microbiota disturbances influence NLRP3 inflammasome activation offers novel perspectives for therapeutic intervention.
Study Design
This review synthesizes evidence from experimental and clinical studies that investigate: 1) alterations in gut microbiota composition following ICH; 2) mechanisms by which microbiota dysbiosis activates the NLRP3 inflammasome via metabolic, neural, and immune pathways; 3) the role of NLRP3 inflammasome activation in secondary WMI after ICH; and 4) emerging therapeutic strategies to modulate these pathways to improve neurological recovery.
The included studies use animal models of ICH and human observational data characterizing gut microbiota changes, inflammatory markers, and imaging-based white matter integrity. Mechanistic insights derive from molecular and cellular analyses of inflammasome activation, neuroinflammation, and neural repair processes.
Key Findings
Gut Microbiota Dysbiosis Following ICH
Post-ICH dysbiosis typically presents as reduced diversity of beneficial commensal bacteria and increased abundance of pathogenic species. Changes include diminished short-chain fatty acid (SCFA)-producing bacteria and overgrowth of endotoxin-rich gram-negative bacteria. This imbalance impairs gut barrier integrity, leading to systemic inflammation and altered metabolite profiles.
Mechanisms Linking Dysbiosis to NLRP3 Inflammasome Activation
Metabolic Pathways:
– SCFAs (e.g., butyrate) normally inhibit inflammasome activation; their reduction removes this brake, facilitating NLRP3 priming.
– Elevated lipopolysaccharides (LPS) from gram-negative bacteria activate Toll-like receptor 4 (TLR4), promoting inflammasome assembly.
– Altered bile acids, lactic acid, trimethylamine-N-oxide (TMAO), and tryptophan metabolites modulate systemic and brain immune responses, influencing inflammasome activity.
Neural Pathways:
– The vagus nerve mediates anti-inflammatory gut-to-brain signaling; impairment after ICH may exacerbate inflammation and inflammasome activation.
– Sympathetic nervous system activation modulates immune cell trafficking and cytokine release, affecting inflammasome status.
Immune Pathways:
– Microglia serve as resident brain immune cells that express NLRP3. Microbial signals modulate microglial priming and inflammasome activation.
– T cell populations influenced by gut microbiota alteration shape neuroinflammatory milieu and inflammasome engagement.
Relationship Between NLRP3 Activation and White Matter Injury
Activated NLRP3 inflammasomes contribute to secondary WMI after ICH through: 1) Disruption of blood-brain barrier (BBB) integrity leading to infiltration of peripheral immune cells and edema; 2) Amplification of neuroinflammation causing oligodendrocyte death and myelin degradation; 3) Impairment of nerve regeneration via inhibition of neural stem cell proliferation and axonal repair.
Potential Treatment Strategies
Emerging therapeutic approaches targeting the gut microbiota-NLRP3 axis include:
– Probiotics, prebiotics, and fecal microbiota transplantation to restore microbial balance.
– Pharmacological inhibitors of NLRP3 inflammasome components (e.g., MCC950).
– Modulation of metabolic pathways using SCFA supplementation or bile acid receptor agonists.
– Vagus nerve stimulation to promote anti-inflammatory signaling.
– Immunomodulatory strategies enhancing regulatory T cell responses.
These approaches aim to attenuate neuroinflammation, protect white matter integrity, and improve functional outcomes.
Expert Commentary
Experts recognize that unraveling the gut-brain inflammatory axis, particularly the pivotal role of NLRP3 inflammasome, opens new avenues for therapeutic intervention after ICH. While preclinical models provide compelling mechanistic evidence, translation to clinical practice necessitates well-designed trials to assess efficacy and safety. Current limitations include patient heterogeneity and complexity of microbiota-host interactions. Nonetheless, integrating microbiome modulation with targeted inflammasome inhibition represents a promising path toward mitigating secondary brain injury and promoting recovery.
Conclusion
The interplay between gut microbiota dysbiosis and NLRP3 inflammasome activation is a critical driver of secondary white matter injury following intracerebral hemorrhage. Disruption of this axis exacerbates neuroinflammation, BBB breakdown, and impairments in neural repair mechanisms. Targeting the gut microbiota-brain axis and NLRP3 inflammasome offers a novel and promising therapeutic strategy to improve neurological outcomes after ICH. Future research should focus on translational studies and optimization of combined gut microbiota and inflammasome-targeted interventions.
References
1. Cai X, Cai X, Xie Q, Xiao X, Li T, Zhou T, Sun H. NLRP3 inflammasome and gut microbiota-brain axis: A new perspective on white matter injury after intracerebral hemorrhage. Neural Regen Res. 2026 Jan 1;21(1):62-80. doi: 10.4103/NRR.NRR-D-24-00917.
2. Yan AW, Charles EJ, Pan X, et al. Gut microbiota and the immune system in ischemic stroke: insight into systemic and cerebral inflammation. Stroke. 2022;53(6):1931-1940.
3. Franchi L, Eigenbrod T, Nunez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10(3):241-247.
4. Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120(7):1183-1196.
5. Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal immune cells. Nat Med. 2016;22(5):516-523.