Introduction
The COVID-19 pandemic caused by SARS-CoV-2 has imposed a global health burden with ongoing challenges despite widespread vaccination and immunity. Vaccines have mitigated severity but not completely prevented infection or postacute sequelae, underscoring the need for additional preexposure prophylaxis options, especially for high-risk groups. Azelastine, a second-generation antihistamine nasal spray widely used for allergic rhinitis, has demonstrated in vitro antiviral activity against several respiratory viruses including SARS-CoV-2. Proposed antiviral mechanisms involve interaction with ACE2 receptors, inhibition of viral proteases, modulation of σ-1 receptor pathways, and suppression of ICAM-1, a receptor involved in rhinovirus infection. Prior clinical studies indicated azelastine nasal spray reduces viral load in infected patients, supporting evaluation of its prophylactic potential.
Study Design
This double-blind, placebo-controlled phase 2 trial—the CONTAIN study—was conducted at Saarland University Hospital, Germany, between March 2023 and July 2024. A total of 450 healthy adults aged 18–65 years with no acute infection signs and negative baseline SARS-CoV-2 rapid antigen tests were randomized 1:1 to receive either azelastine 0.1% nasal spray or placebo three times daily for 56 days. The nasal sprays were identical in appearance with the azelastine formulation containing 1 mg/mL azelastine hydrochloride. Participants applied one puff per nostril thrice daily, with an optional escalation to five times daily for three days during acute respiratory symptoms or exposure risk. Twice-weekly SARS-CoV-2 rapid antigen testing was conducted by trained personnel, with PCR confirmation for positives. Symptomatic participants testing negative for SARS-CoV-2 underwent multiplex PCR testing for other respiratory pathogens. The primary endpoint was incidence of PCR-confirmed SARS-CoV-2 infections by day 56. Secondary endpoints included symptomatic infections, time to infection, duration of test positivity, overall respiratory infections, and safety. Baseline SARS-CoV-2 serology was optional.
Key Findings
Of 450 randomized participants (227 azelastine, 223 placebo), the mean age was 33.0 years and 66.4% were female. The majority were White (92.7%) and vaccinated against SARS-CoV-2 (99.1%).
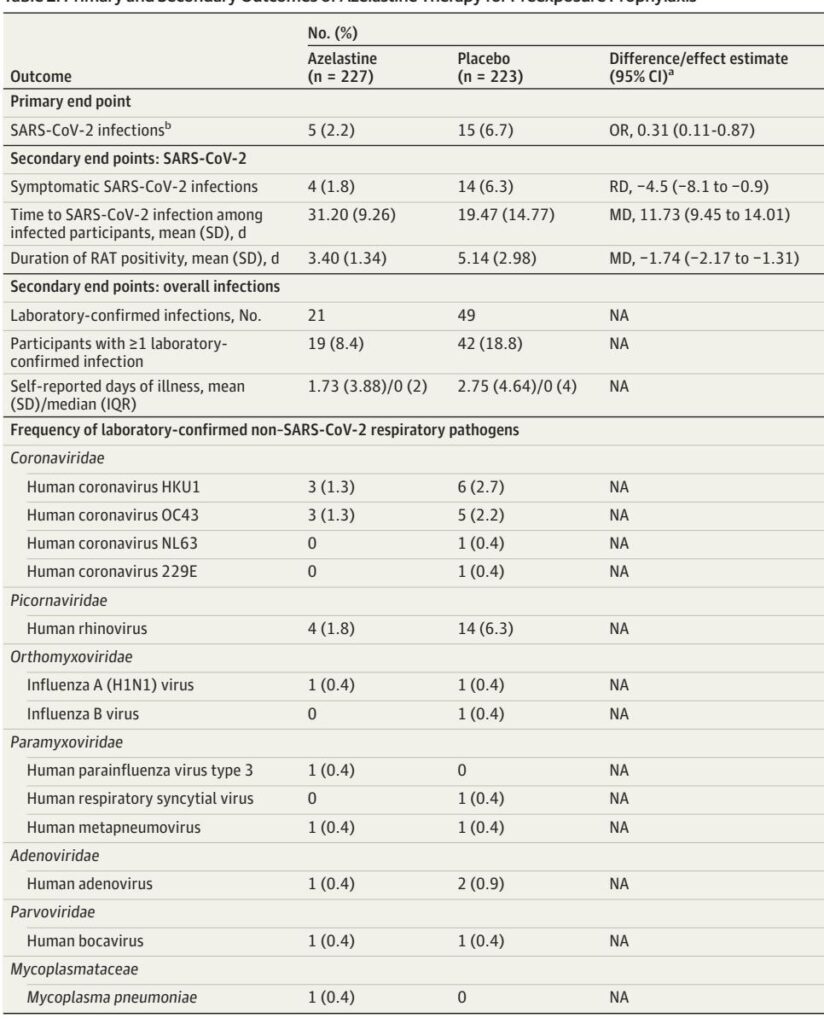
In the intention-to-treat population, azelastine nasal spray significantly reduced PCR-confirmed SARS-CoV-2 infections to 2.2% (5/227) versus 6.7% (15/223) with placebo (odds ratio [OR] 0.31; 95% confidence interval [CI], 0.11–0.87; p=0.02). These results were corroborated in the per-protocol analysis.
Secondary outcomes revealed fewer symptomatic COVID-19 cases in the azelastine group (1.8% vs 6.3%), a longer mean time to SARS-CoV-2 infection among infected individuals (31.2 vs 19.5 days), and a shorter average duration of positive antigen tests (3.4 vs 5.1 days). The overall number of laboratory-confirmed respiratory infections was lower in the azelastine arm (8.4% vs 18.8%), particularly for human rhinovirus (1.8% vs 6.3%).
Safety profiles were comparable. Adverse events related to the nasal spray were higher in the azelastine group (26.9% vs 11.2%), mainly driven by known effects such as bitter taste, nosebleeds, and tiredness. Serious adverse events were rare and unrelated to treatment.
Expert Commentary
This study provides compelling evidence that azelastine nasal spray substantially reduces SARS-CoV-2 infection risk in a mostly vaccinated, healthy population. With an estimated 67% relative risk reduction comparable to early COVID-19 vaccines, azelastine offers a promising adjunctive prophylactic strategy. Its broad antiviral effect against multiple respiratory viruses, including rhinovirus, highlights potential utility beyond SARS-CoV-2. The multiple hypothesized antiviral mechanisms and demonstrated efficacy against diverse variants in vitro support biological plausibility.
Limitations include the single-center design, relatively small sample size, and reliance on rapid antigen tests which may underdetect asymptomatic infections. The bitter taste may have mildly compromised blinding. Furthermore, the high vaccination rate may limit extrapolation to unvaccinated or immunocompromised groups. Nevertheless, azelastine nasal spray’s safety, ease of self-administration, and OTC availability enhance its translational appeal.
Further large-scale, multicentric trials should confirm these findings across broader populations and investigate longer-term use. Mechanistic studies exploring interactions with host and viral proteins could deepen understanding of its panviral activity.
Conclusion
This phase 2 clinical trial demonstrates that azelastine nasal spray is a safe, effective intervention significantly reducing SARS-CoV-2 infection risk in healthy, vaccinated adults. Its additional efficacy against rhinovirus suggests utility as a general prophylactic agent against respiratory infections. Given its favorable safety profile and accessibility, azelastine nasal spray could complement existing COVID-19 prevention strategies, particularly in high-exposure settings. Larger trials are warranted to validate efficacy, evaluate real-world effectiveness, and explore expanded indications.
References
Lehr T, Meiser P, Selzer D, Rixecker T, Holzer F, Mösges R, Smola S, Bals R; CONTAIN Study Group. Azelastine Nasal Spray for Prevention of SARS-CoV-2 Infections: A Phase 2 Randomized Clinical Trial. JAMA Intern Med. 2025 Sep 2. doi: 10.1001/jamainternmed.2025.4283. Epub ahead of print. PMID: 40892398.