Neurodegenerative diseases (NDs), including Alzheimer’s disease (AD), Parkinson’s disease (PD), stroke, amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), multiple sclerosis (MS), and epilepsy, impose substantial morbidity and mortality worldwide. These disorders are characterized by progressive neuronal dysfunction, involving complex pathogenic mechanisms such as protein misfolding and aggregation, mitochondrial dysfunction, oxidative stress, and chronic neuroinflammation. Despite extensive research, current therapies mainly provide symptomatic relief and fail to halt disease progression. Therefore, innovative therapeutic strategies are urgently needed.
Exosomes—small extracellular vesicles (30–100 nm) derived from diverse cell types—have emerged as potent mediators of intercellular communication in the central nervous system (CNS). Their intrinsic properties, including the ability to traverse the blood–brain barrier (BBB), low immunogenicity, and natural cargo-carrying capabilities, position exosomes as attractive candidates for delivering therapeutic molecules and serving as biomarkers for early diagnosis of NDs.
This article reviews the therapeutic potential of exosomes in neurodegenerative disorders and details the emerging strategies to enhance their targeting and efficacy through genetic, chemical, and nanomaterial modifications. We highlight recent preclinical findings, address current limitations, and discuss future outlooks for clinical translation.
Exosomes in Neurodegenerative Diseases
Exosomes originate intracellularly via endosomal processing and are released into extracellular spaces where they carry proteins, lipids, mRNAs, microRNAs (miRNAs), and other bioactive molecules. Exosomes influence recipient cells by transferring these cargos, thereby modulating immune responses, synaptic function, and cellular homeostasis.
In AD, exosomes contribute to both pathological propagation and clearance of amyloid-beta (Aβ) and tau aggregates. Mesenchymal stem cell-derived exosomes (MSC-exosomes) loaded with therapeutic miRNAs (e.g., miR-223 targeting PTEN) have demonstrated neuroprotective effects, reducing inflammation and neuronal apoptosis in animal models, effectively improving cognitive outcomes. Engineered exosomes decorated with rabies virus glycoprotein (RVG) peptides exhibit enhanced BBB penetration and microglial uptake, further amplifying therapeutic delivery.
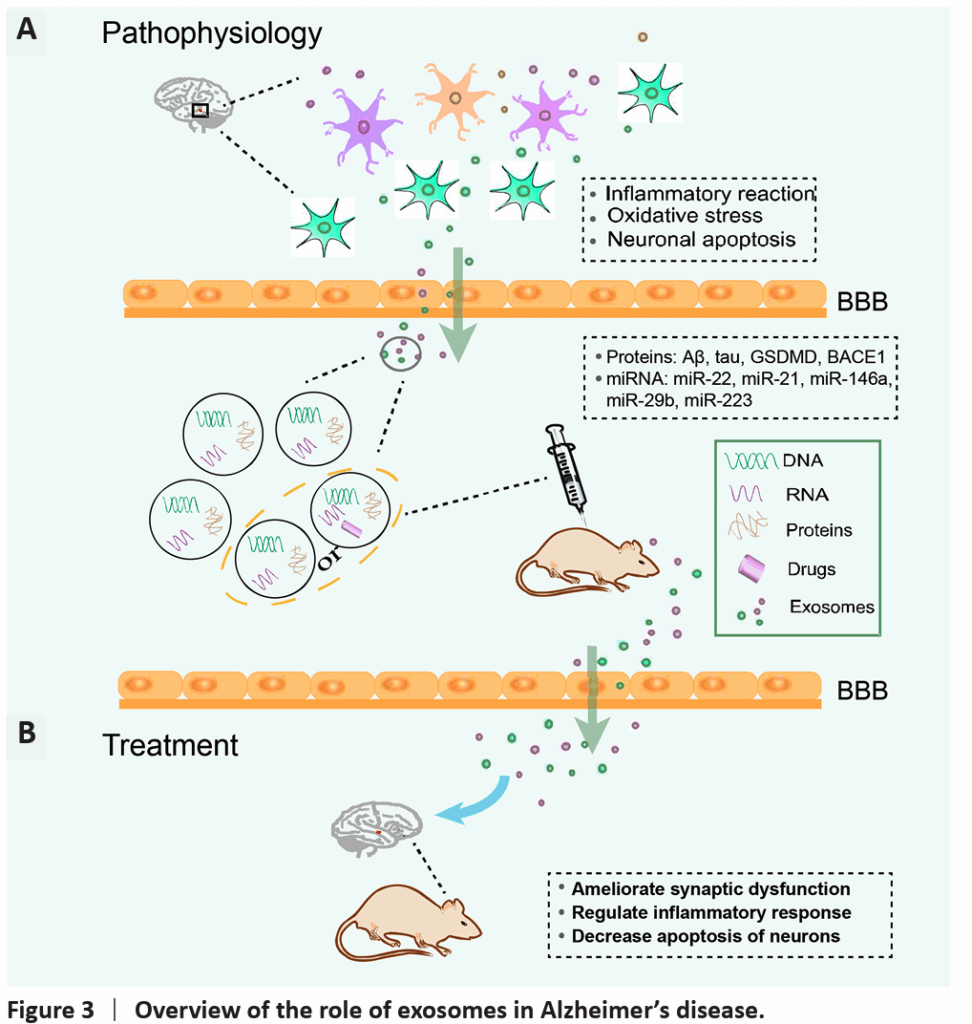
In PD, exosomes offer a vehicle for delivering antioxidative enzymes, neurotrophic factors, and siRNAs targeting α-synuclein aggregates. Numerous studies show that administration of MSC-exosomes mitigates dopaminergic neuron loss, reduces neuroinflammation, and restores motor function in rodent models. Exosomes carrying miR-133b promote neurite outgrowth, enhancing synaptic plasticity.
Stroke management benefits from exosomal delivery of angiogenic and neurogenic factors, such as miR-210 and miR-126 from human urine or bone marrow-derived stem cells. Exosomes reduce infarct size, inhibit neuronal apoptosis, and modulate microglial activation, expediting functional recovery. Importantly, exosomes’ endogenous nature favors targeting and evasion of immune clearance compared to synthetic nanoparticles.
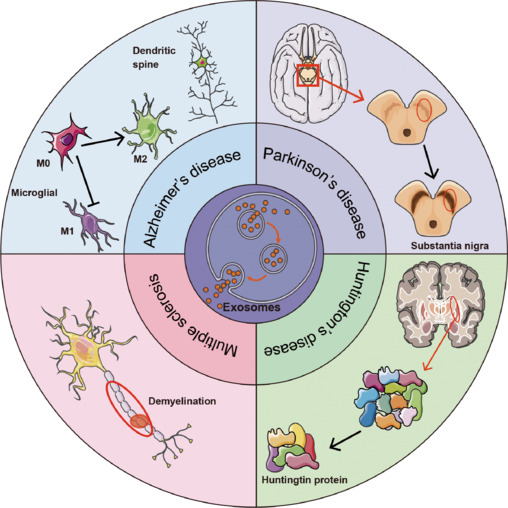
Beyond these, exosomes have shown promising effects in ALS, HD, MS, and epilepsy by modulating neuroinflammation, oxidative stress, and pathogenic protein aggregation, indicating their broad applicability across diverse NDs.
Modification of Exosomes to Enhance Targeting and Therapeutic Efficiency
While natural exosomes exhibit significant therapeutic capabilities, their lack of inherent target-cell specificity limits clinical application due to uptake by off-target tissues and reduced efficacy. Hence, emerging technologies focus on engineering exosomes to optimize targeting, loading, and delivery.
Genetic Engineering
Genetic modification entails transfecting exosome-producing cells with plasmids encoding fusion proteins that append targeting ligands or peptides onto exosomal membrane proteins (e.g., Lamp2b). This approach enables surface display of ligands such as RVG, which enhances CNS targeting, or cardiac-specific peptides for heart diseases.
Examples include CD9-HuR fusion to promote siRNA loading, and Fe65 peptide fusion to target amyloid precursor proteins, facilitating targeted autophagy induction in AD models. Genetic engineering allows precise ligand incorporation but requires complex manufacturing and high cost.
Chemical Modifications
Chemical approaches include both covalent and non-covalent surface functionalization. Covalent modification mostly employs bioorthogonal click chemistry, utilizing azide-alkyne cycloaddition reactions to attach targeting moieties or nanoparticles. For instance, exosomes conjugated with superparamagnetic iron oxide nanoparticles (SPIONs) and neuropilin-1 targeting peptides demonstrate improved glioma targeting and imaging capability.
Non-covalent strategies utilize electrostatic interactions, ligand-receptor binding, hydrophobic fusion with liposomes, aptamer incorporation, and CP05 peptide anchoring to the CD63 exosomal tetraspanin. These allow reversible surface modifications with minimal impact on exosome integrity. For example, RVG peptides tethered via DOPE-NHS linkers increase brain delivery of MSC-exosomes.
Nanomaterial Integration
Integration with nanomaterials broadens exosome functionality. Metal-organic frameworks and gold nanoparticles incorporated onto or within exosomes facilitate drug loading, tracking, and controlled release. Nanomaterial-enhanced exosomes improve drug stability, targeted accumulation, and real-time imaging, supporting theranostic applications.
Researchers have successfully used nanomaterial-modified exosomes to deliver hydrophobic drugs and to scavenge pathological proteins like α-synuclein in PD models, highlighting their utility in overcoming drug solubility challenges and enhancing therapeutic efficacy.
Limitations and Challenges
Despite promising preclinical results, several barriers hinder the clinical translation of exosome-based therapies. Variability in exosome source, heterogeneity in cargo composition, and challenges in large-scale purification complicate standardization. Exosomes display short systemic half-lives and rapid clearance, necessitating optimization for sustained therapeutic effects.
Potential adverse effects due to unintended interactions with non-target cells or transfer of pathological proteins require rigorous safety evaluation. Ethical issues arise with stem cell-derived exosomes, especially regarding source material and regulatory oversight.
Technological challenges include efficient cargo loading, stability during storage, and developing methods for high-yield, reproducible production. Advances in characterization and imaging techniques are needed to accurately track exosome biodistribution and fate in vivo.
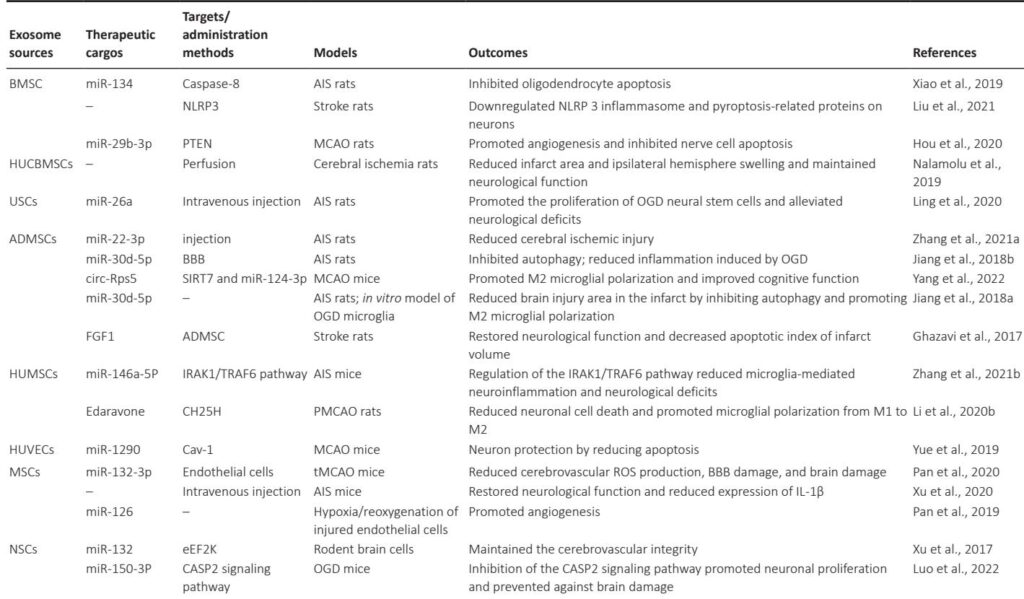
Summary of various exosomes in other ND therapeutics
| Diseases | Exosome sources | Therapeutic cargos | Targets/administration methods | Models | Outcomes | References |
|---|---|---|---|---|---|---|
| ALS | ADMSCs | – | SOD1 | SOD1-G93A mice | Reduced glial cell activation in mice | Bonafede et al., 2020 |
| MSCs | miR-467f; miR-466q |
MAPK signaling pathway | SOD1-G93A mice | Downregulated TNF, IL-6 and IL-1β expression Inhibited Map3k8 and Mk2 and thus the pro-inflammatory phenotype of microglia |
Giunti et al., 2021 | |
| NSCs | miR-124a | Striata | SOD1-G93A mice | Reduced GLT1 protein expression in mice | Morel et al., 2013 | |
| HD | ADMSCs | – | Htt | R6/2 transgenic HD mice | Upregulated PGC-1 levels to improve mitochondrial dysfunction | Lee et al., 2016b |
| HEK 293 cells | miR-124 | Striata | R6/2 transgenic HD mice | Reduced expression of REST protein of target genes | Lee et al., 2017 | |
| Serum | – | Intravenous injection | R6/2 transgenic HD mice | Improved weight loss and survival in HD mice | Lee et al., 2021 | |
| MSCs | hsiRNA | Striata | Wild-type mice | Reduced Htt expression | Didiot et al., 2016 | |
| MS | NSCs | Montelukast | GPR17 | Cuprizone-treated mice | Promoted myelination | Xiao et al., 2022 |
| Bryostatin-1 | GPR17 | Cuprizone-treated mice | Promoted myelination | Wu et al., 2022 | ||
| Dendritic cells | IFNγ | Intranasally | MS mice | Promoted myelination in the CNS | Pusic et al., 2014 | |
| ADMSCs | – | Intranasally | EAE mice | Significantly reduced CNS tissue lesions | Fathollahi et al., 2021 | |
| MSCs | Carboxylic acid functionalized LJM-3064 aptamers | Amine groups on the surface of exosomes | C57BL/6 mice | Reduced area of demyelinating lesions of the central nervous system | Hosseini Shamili et al., 2019 | |
| EP | BMSCs | – | Hippocampus | LPS EP mice | Weakened microglial activation, and reduced hippocampal neuroinflammation | Long et al., 2017 |
Conclusion and Future Perspectives
Exosomes represent a novel and versatile platform for the diagnosis and treatment of neurodegenerative diseases due to their inherent ability to cross the BBB and mediate intercellular communication. Stem cell-derived exosomes exhibit potent regenerative, immunomodulatory, and neuroprotective effects in multiple ND models. Furthermore, surface modifications via genetic engineering, chemical conjugation, and nanomaterial incorporation substantially improve targeting specificity and therapeutic outcomes.
To realize their clinical potential, future research must focus on addressing production scalability, purification standardization, safety profiling, and controlled cargo loading. Interdisciplinary efforts integrating nanotechnology, molecular biology, and clinical neuroscience will be crucial.
Ultimately, engineered exosome-based platforms hold promise as precision medicine tools, offering hope for effective therapies to modify the course of devastating neurodegenerative disorders.
Reference
Chen H, Li N, Cai Y, Ma C, Ye Y, Shi X, Guo J, Han Z, Liu Y, Wei X. Exosomes in neurodegenerative diseases: Therapeutic potential and modification methods. Neural Regen Res. 2026 Feb 1;21(2):478-490. doi: 10.4103/NRR.NRR-D-24-00720 . Epub 2024 Oct 22. PMID: 40326981 ; PMCID: PMC12220696 .