Highlight
- Genetically modified pig-to-baboon liver xenotransplantation demonstrates survival beyond 4 weeks as a bridge therapy for acute liver failure.
- Xenotransplantation research must carefully balance scientific progress with ethical considerations, favoring academic and government oversight over commercial dominance.
- Regulatory frameworks, including expert committees, are critical to ensure patient safety and equitable clinical implementation.
- Public opinion studies reveal broad acceptance of xenotransplantation across diverse populations, emphasizing need to focus on research direction and regulation rather than societal hesitation.
Study Background
Chronic and acute liver diseases continue to contribute significantly to global morbidity and mortality. Liver transplantation remains the definitive treatment for end-stage liver disease and acute liver failure, but shortages in human donor organs cause critical delays or inability to treat many patients. This unmet clinical need has stimulated research into alternative organ sources. Xenotransplantation, the transplantation of organs across species—primarily from genetically modified pigs to primates or humans—has emerged as a promising frontier. The genetic modification of donor pigs aims to reduce immunologic rejection and improve organ compatibility, potentially overcoming the organ shortage crisis.
Study Design and Preclinical Advances
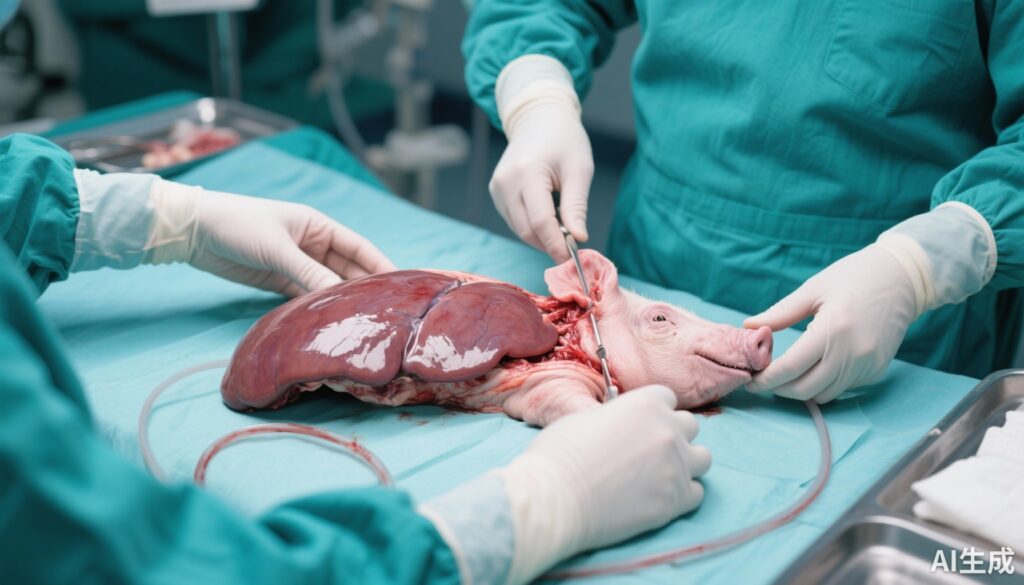
Preclinical investigations have increasingly leveraged transgenic pig models to transplant livers into baboons, simulating the immunologic and physiological challenges of cross-species transplant. Using advanced gene-editing technologies, pigs have been engineered to knock out key carbohydrate antigens and express human complement regulatory and anticoagulant proteins to attenuate hyperacute rejection and coagulation abnormalities. These models have achieved survival periods exceeding four weeks, which marks a significant improvement in graft survival compared with earlier attempts. This time window is considered vital for bridging patients with acute liver failure to human donor availability or recovery.
Key Findings and Clinical Implications
The observation that pig-to-baboon liver xenotransplants can sustain life for over four weeks underscores the potential for clinical translation. In cases of acute liver failure—often fulminant and rapidly fatal without timely transplantation—such bridging could be lifesaving. The clinical application envisaged is not a permanent liver replacement but a temporizing measure allowing stabilization or transition to a human allograft. Experiences from cardiac and renal xenotransplantation clinical trials using genetically modified pig organs complement these findings, suggesting incremental progress towards feasibility and safety.
However, xenotransplantation still faces numerous immunologic, infectious, and ethical hurdles. Although progress has been made on modulating rejection and clotting, long-term graft acceptance remains unproven. Infectious risk, particularly zoonoses, necessitates rigorous oversight. Furthermore, the complex technical and bioethical issues call for structured governance.
Scientific and Ethical Considerations
A standout perspective recommends that xenotransplantation research should remain primarily in the academic and publicly regulated sectors rather than being driven predominantly by profit-oriented pharmaceutical companies. Maintaining rigorous oversight through government-coordinated consortia and expert committees is essential to prevent commodification of genetically engineered organs and to guarantee equitable access.
Ethical frameworks should foster transparency, patient safety, and equitable availability. The recent formation in 2024 of the Council of Europe’s Expert Committee on Xenotransplantation, alongside the FDA’s milestone approval for cardiac xenotransplantation in 2022, exemplifies institutional recognition of the need for robust regulatory pathways. Such committees must carefully balance scientific innovation with patient protection and societal considerations.
Public Perception and Societal Impact
Contrary to assumptions of public resistance, studies from pioneering research groups show generally favorable attitudes towards xenotransplantation across populations varying in age, culture, religion, and socioeconomic status. This positive reception suggests public acceptance should not be viewed as the primary barrier to clinical adoption. Instead, focus should shift to guiding research prioritization, establishing ethical frameworks, and creating regulatory standards to navigate complex scientific and social challenges.
Conclusion
Xenotransplantation stands at a pivotal juncture. Significant strides have brought the prospect of genetically modified pig organ transplantation closer to clinical reality, particularly as a bridge therapy for acute liver failure where human donors are unavailable. However, to translate these advances into safe, ethical, and accessible therapies, coordinated regulation, academic stewardship, and public health policy must underpin future research and clinical applications.
Without such focus, the field risks stagnation under repeated skepticism, echoing Sir Roy Calne’s cautionary remark that xenotransplantation remains perpetually a future promise. With careful governance, xenotransplantation could indeed become a transformative element in organ transplantation medicine.