Introduction
Stroke remains a leading cause of death and disability worldwide, disproportionately affecting individuals with diabetes mellitus. Notably, diabetic peripheral neuropathy (DPN), a common microvascular complication of diabetes, has been linked to an increased risk of ischemic stroke. However, the specific etiological pattern connecting DPN to stroke subtypes remains unclear. Understanding whether DPN is predictive of certain ischemic stroke mechanisms, such as cerebral small-vessel disease (SVD), holds promise for targeted prevention strategies and improved management of cerebrovascular risk in diabetic populations.
Study Design and Methods
A retrospective observational cohort study was conducted at the Centre Hospitalier Andree Rosemon, Cayenne, French Guiana, including 226 patients hospitalized with ischemic stroke between January 2020 and December 2023. Patient inclusion was based on ICD10 codes for ischemic stroke. DPN diagnosis was established by endocrinologists using International Working Group on the Diabetic Foot (IWGDF) criteria, including monofilament testing, ankle reflexes, and vibration threshold assessments.
The primary endpoint was identifying any association between DPN and stroke etiology based on the ASCOD classification system (Atherosclerosis, Small-vessel disease, Cardiac pathology, Other causes, Dissection). Secondary endpoints assessed relationships between DPN and age, sex, stroke severity (NIHSS and mRS scores), stroke recurrence, and one-year mortality. Statistical analyses included univariate and multivariate logistic regression to determine odds ratios (ORs) and adjusted odds ratios (aORs) for associations.
Key Findings
The cohort had a mean age of 64.14 years and a male predominance (62.4%). DPN was found in 5.8% of patients overall, representing 14.9% of those with diabetes. DPN prevalence was higher among females and correlated with elevated HbA1c and CHA2DS2-VASc scores, indicating greater cardiovascular risk burden.
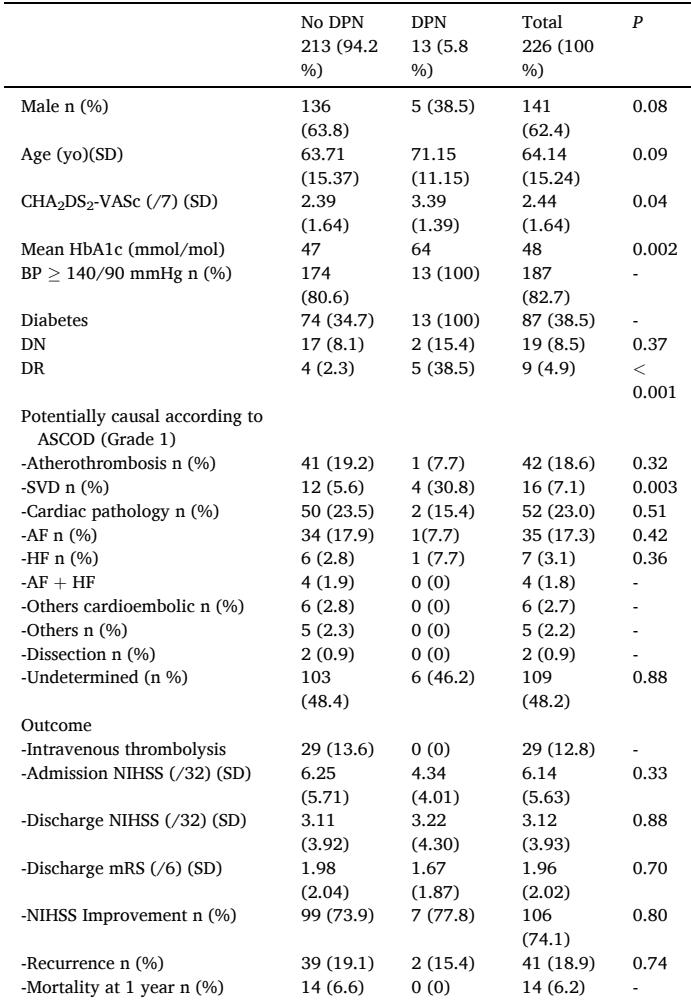
Table 3. Multivariate analysis of factors associated with small vessel disease.
| Empty Cell | SVD aOR (CI 95) |
|---|---|
| Age 60 yo | 0.98 (0.82;1.18) |
| Male | 0.48 (0.16;1.47) |
| CHA2DS2-VASc2 | 5.45 (0.63;47.10) |
| Diabetes | 0.98 (0.29;3.42) |
| DPN | 5.98 (1.17;22.14) |
Table 4. Factors associated with all phenotypes of small-vessel disease.
| All phenotypes of SVD n = 39 | OR (CI 95) | aOR (CI 95) |
|---|---|---|
| Age ≥ 60 yo | 0.98 (0.89;1.09) | 0.98 (0.87;1.10) |
| Male | 0.84 (0.42;1.70) | 0.87 (0.41;1.83) |
| CHA2DS2-VASc ≥ 2 | 0.70 (0.34;1.44) | |
| BP ≥ 140/90 mmHg | 2.86 (0.83;9.81) | |
| Diabetes | 1.00 (0.49;2.03) | 0.64 (0.27;1.48) |
| DPN | 4.67 (1.47;14.79) | 6.19 (1.70;22.58) |
| DN | 1.79 (0.60;5.29) | |
| DR | 2.53 (0.60;10.71) |
Crucially, DPN was significantly associated with ischemic strokes caused by SVD. The unadjusted OR was 7.44 (95% CI 2.00–27.70), and the association remained significant after adjustment for confounders with an aOR of 5.08 (95% CI 1.17–22.14). The attributable fraction of SVD strokes among patients with DPN was 86.6%, and the population attributable fraction was estimated at 21.6%, underscoring the impact of DPN as an indicator of microvascular brain disease. No significant associations were observed between DPN and other stroke etiologies, nor with stroke severity, recurrence, or mortality within one year.
Discussion and Expert Commentary
This study’s findings provide compelling evidence that DPN is a strong clinical marker for cerebral SVD in diabetic stroke patients, aligning with shared pathophysiological features of microangiopathy in both conditions. The microvascular damage inherent in DPN appears to parallel cerebral small vessel alterations seen in lacunar infarcts.
Given that SVD contributes not only to lacunar stroke but also to vascular cognitive impairment and dementia, early identification of DPN offers a valuable window for preventive interventions targeting glycemic control, hypertension, and other modifiable risk factors. The observed prevalence of DPN in stroke patients was consistent with previous epidemiological data, though retrospective assessment may underestimate true prevalence due to asymptomatic neuropathy.
Mechanistically, hyperglycemia-induced pathways—such as advanced glycation end-products, polyol and protein kinase C activation—along with oxidative stress and mitochondrial dysfunction, contribute to nerve fiber injury and microvascular endothelial damage in both DPN and SVD. The interplay between systemic metabolic derangements and microvascular pathology reinforces the concept of a “diabetic panvascular disease,” integrating peripheral and cerebral microangiopathies.
Limitations include retrospective design and potential underdiagnosis of DPN due to incomplete neurological evaluations in all stroke patients. Furthermore, establishing a causal link remains challenging, warranting prospective studies with standardized DPN assessment and longitudinal follow-up to evaluate stroke outcomes and recurrence risks.
Conclusion
Diabetic peripheral neuropathy is significantly associated with cerebral small-vessel disease and lacunar stroke in diabetic patients. DPN may serve as a practical clinical marker for identifying individuals at higher risk for microvascular cerebral ischemic events, facilitating earlier and targeted preventive strategies. Integrating comprehensive neuropathic evaluation into diabetic care could improve cerebrovascular risk stratification and ultimately reduce stroke burden in this vulnerable population.
References
- Feigin VL, Stark BA, CO J, Roth GA, Bisignano C, Abady GG, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(7):795-820.
- Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME, Hennerici MG. The ASCOD phenotyping of ischemic stroke (Updated ASCO Phenotyping). Cerebrovasc Dis. 2013;36(1):1-5.
- Brownrigg JRW, De Lusignan S, McGovern A, et al. Peripheral neuropathy and the risk of cardiovascular events in type 2 diabetes mellitus. Heart. 2014;100(23):1837-43.
- Selvarajah D, Kar D, Khunti K, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7(12):938-948.

